Integration of Advanced Technologies
The herg screening market is witnessing a transformation due to the integration of advanced technologies such as high-throughput screening and automated testing systems. These innovations enhance the efficiency and accuracy of herg screening processes, allowing for faster drug development timelines. In Spain, the adoption of these technologies is expected to increase, driven by the need for more reliable and reproducible results. The market for automated herg screening solutions is anticipated to grow by approximately 10% annually, as pharmaceutical companies seek to streamline their testing protocols. This technological evolution not only improves the quality of testing but also reduces costs associated with drug development, thereby positioning the herg screening market as a critical component in the pharmaceutical industry's quest for innovation.
Increased Investment in Drug Development
Investment in drug development is a key driver for the herg screening market in Spain. As pharmaceutical companies allocate more resources towards research and development, the need for comprehensive safety assessments, including herg screening, becomes paramount. In recent years, Spain has seen a rise in funding for biotech and pharmaceutical ventures, with investments reaching over €1 billion in 2025. This influx of capital is likely to bolster the herg screening market, as companies seek to ensure that their products meet safety standards before reaching the market. Furthermore, the competitive landscape encourages firms to adopt rigorous testing protocols, thereby enhancing the overall quality of drug development. Consequently, the herg screening market is poised for growth as it becomes an integral part of the drug development pipeline.
Rising Demand for Cardiac Safety Testing
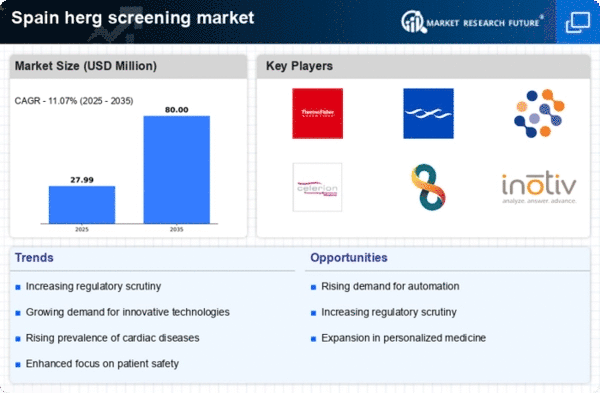
The herg screening market in Spain is experiencing a notable increase in demand for cardiac safety testing. This trend is largely driven by the growing awareness of the risks associated with drug-induced arrhythmias. Pharmaceutical companies are increasingly prioritizing cardiac safety in their drug development processes, leading to a surge in the adoption of herg screening methods. In 2025, the market is projected to reach approximately €150 million, reflecting a compound annual growth rate (CAGR) of around 8% over the next five years. This growth is indicative of the industry's commitment to ensuring patient safety and compliance with stringent regulatory standards. As a result, the herg screening market will expand significantly, with more innovative testing solutions being developed to meet the evolving needs of the pharmaceutical sector..
Regulatory Pressure for Safety Assessments
Regulatory bodies in Spain are imposing stricter guidelines regarding safety assessments for new pharmaceuticals, which is significantly impacting the herg screening market. The emphasis on ensuring that drugs do not pose risks of cardiac arrhythmias has led to an increased focus on herg screening as a mandatory step in the drug approval process. In 2025, it is estimated that compliance with these regulations will drive a 15% increase in the demand for herg screening services. Pharmaceutical companies are now more inclined to invest in comprehensive herg testing to avoid potential liabilities and ensure market access. This regulatory pressure not only enhances the credibility of the herg screening market but also fosters innovation in testing methodologies, as companies strive to meet the evolving standards set forth by regulatory authorities.
Growing Focus on Patient-Centric Approaches
The herg screening market is increasingly influenced by a growing focus on patient-centric approaches within the healthcare sector. As stakeholders prioritize patient safety and treatment efficacy, the demand for thorough herg screening becomes more pronounced. In Spain, healthcare providers and pharmaceutical companies are recognizing the importance of tailoring drug therapies to individual patient profiles, which necessitates rigorous safety evaluations. This shift is expected to contribute to a market growth rate of approximately 12% over the next few years. By integrating herg screening into personalized medicine strategies, the industry aims to mitigate risks associated with drug therapies, thereby enhancing patient outcomes. Consequently, the herg screening market is likely to evolve in tandem with these patient-centric initiatives, reinforcing its role in the broader healthcare landscape.