Increased Healthcare Expenditure
Spain's healthcare expenditure has been steadily increasing, which positively influences the hepatitis test-solution-diagnosis market. In recent years, the government has allocated a larger portion of its budget to healthcare, with spending reaching approximately €150 billion in 2025. This financial commitment facilitates the procurement of advanced diagnostic technologies and testing solutions. As healthcare facilities upgrade their capabilities, the demand for hepatitis testing solutions is likely to rise. Furthermore, increased funding for public health initiatives aimed at combating viral hepatitis enhances awareness and accessibility of testing services. This trend indicates a robust market environment where investments in healthcare infrastructure directly correlate with the growth of the hepatitis test-solution-diagnosis market.
Advancements in Molecular Diagnostics
Advancements in molecular diagnostics are revolutionizing the hepatitis test-solution-diagnosis market. Innovative technologies, such as PCR and next-generation sequencing, are enabling more accurate and sensitive detection of hepatitis viruses. In Spain, the adoption of these advanced diagnostic methods is increasing, as they provide rapid and reliable results, which are crucial for effective patient management. The market for molecular diagnostics is expected to expand significantly, with projections indicating a growth rate of approximately 10% annually. This trend suggests that healthcare providers are prioritizing precision medicine, which is likely to enhance the overall quality of hepatitis care. As molecular diagnostics become more accessible, they are expected to play a pivotal role in the future of hepatitis testing.
Rising Incidence of Hepatitis Infections
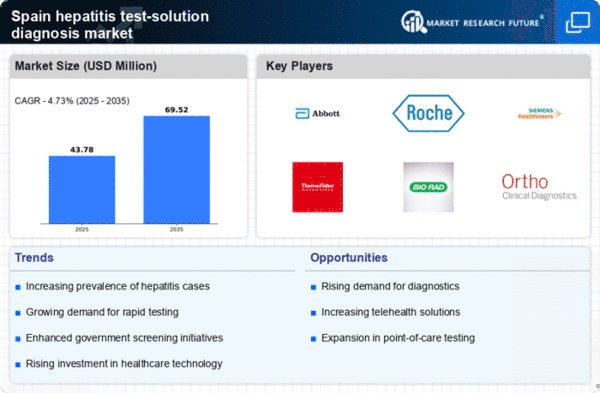
The increasing incidence of hepatitis infections in Spain is a critical driver for the hepatitis test-solution-diagnosis market. Recent data indicates that the prevalence of hepatitis B and C is on the rise, with estimates suggesting that approximately 0.5-1.0 million individuals are living with chronic hepatitis B and around 200,000 with chronic hepatitis C. This growing patient population necessitates enhanced diagnostic solutions, thereby propelling market growth. Healthcare providers are increasingly focusing on early detection and treatment, which further stimulates demand for innovative testing solutions. The urgency to address these infections is underscored by the potential for severe health complications, such as liver cirrhosis and hepatocellular carcinoma. This makes effective diagnosis paramount in Spain's healthcare landscape.
Growing Demand for Rapid Testing Solutions
The demand for rapid testing solutions is significantly shaping the hepatitis test-solution-diagnosis market. In Spain, healthcare providers are increasingly adopting point-of-care testing methods that offer quick results, often within minutes. This shift is driven by the need for timely diagnosis and treatment, particularly in emergency settings. Rapid tests not only improve patient outcomes but also enhance the efficiency of healthcare delivery. The market for rapid hepatitis tests is projected to grow at a CAGR of around 8% over the next few years, reflecting the urgency for immediate diagnostic solutions. As healthcare systems evolve, the integration of rapid testing into routine practice is likely to become a standard, further propelling market growth.
Regulatory Support for Diagnostic Innovations
Regulatory support for diagnostic innovations is a vital driver for the hepatitis test-solution-diagnosis market. In Spain, regulatory bodies are actively promoting the development and approval of new diagnostic tests, which encourages investment in research and development. The streamlined approval processes for innovative testing solutions are likely to accelerate the introduction of advanced hepatitis diagnostics into the market. This supportive regulatory environment fosters collaboration between public health authorities and private sector companies, enhancing the overall landscape for hepatitis testing. As new technologies emerge, the market is expected to benefit from increased competition and improved testing options, ultimately leading to better health outcomes for patients.