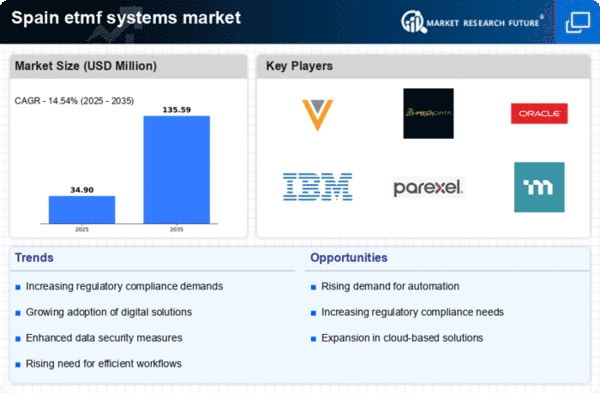
Emphasis on Cost Reduction Strategies
In the current economic climate, organizations in Spain are increasingly focused on reducing operational costs. The etmf systems market is benefiting from this trend as companies recognize the potential for cost savings through the implementation of electronic trial master files. By transitioning from paper-based systems to digital solutions, organizations can significantly lower expenses related to document storage, retrieval, and management. It is estimated that companies can save up to 30% in operational costs by adopting etmf systems. This emphasis on cost reduction is likely to propel the growth of the etmf systems market in Spain, as more organizations seek to optimize their resources.
Rising Need for Enhanced Collaboration
The etmf systems market in Spain is experiencing growth due to the rising need for enhanced collaboration among stakeholders in clinical trials. As the industry becomes more interconnected, the ability to share information seamlessly is paramount. Electronic trial master files facilitate real-time access to critical documents, enabling better communication between sponsors, CROs, and regulatory bodies. This collaborative approach not only improves efficiency but also ensures compliance with regulatory requirements. The increasing emphasis on teamwork and information sharing is likely to drive the adoption of etmf systems, positioning them as essential tools for successful clinical trial management in Spain.
Growing Demand for Data Management Solutions
The increasing complexity of clinical trials and the need for efficient data management are driving the growth of the etmf systems market. Organizations are seeking solutions that streamline data collection, storage, and retrieval processes. This demand is reflected in the market, which is projected to grow at a CAGR of approximately 12% over the next five years. As companies strive to enhance operational efficiency, the adoption of etmf systems becomes crucial. The ability to manage vast amounts of data effectively not only improves compliance but also accelerates the decision-making process. Consequently, the growing demand for robust data management solutions is a key driver for the etmf systems market in Spain.
Increased Focus on Patient-Centric Approaches
The shift towards patient-centric clinical trials is influencing the etmf systems market in Spain. Organizations are recognizing the importance of incorporating patient feedback and preferences into trial designs. This focus on patient engagement necessitates the use of electronic trial master files that can efficiently manage and analyze patient-related data. By leveraging etmf systems, companies can enhance their ability to adapt trials based on patient insights, ultimately improving outcomes. The growing emphasis on patient-centricity is likely to drive the adoption of etmf systems, as organizations seek to align their processes with the evolving expectations of patients and regulatory authorities.
Regulatory Pressure for Digital Transformation
Regulatory bodies in Spain are increasingly advocating for the digital transformation of clinical trial processes. This push for modernization is significantly impacting the etmf systems market, as organizations are compelled to adopt electronic solutions to meet compliance standards. The Spanish Agency of Medicines and Medical Devices has outlined guidelines that encourage the use of digital systems for document management. As a result, companies are investing in etmf systems to align with these regulatory expectations. This regulatory pressure is likely to accelerate the growth of the etmf systems market, as organizations strive to remain compliant while enhancing their operational capabilities.