

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
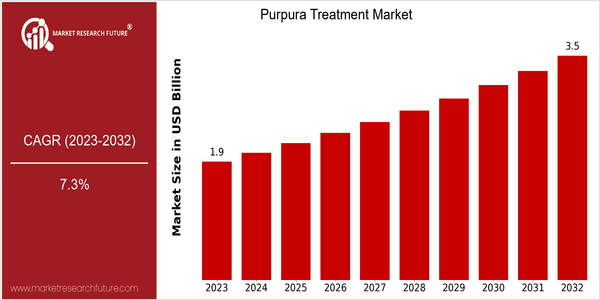
Market Size Snapshot
| Year | Value |
|---|---|
| 2023 | USD 1.86 Billion |
| 2032 | USD 3.5 Billion |
| CAGR (2024-2032) | 7.3 % |
Note – Market size depicts the revenue generated over the financial year
The global purpura treatment market is estimated to be worth USD 1.86 billion in 2023 and is likely to reach USD 3.5 billion by 2032, registering a CAGR of 7.3% from 2024 to 2032. This growth is expected to be driven by an increase in the awareness of the disease, advancements in medical technology, and the rising prevalence of related disorders. The increasing focus on the development of personalized medicine and the introduction of new therapeutic solutions is also expected to drive the market. The development of novel therapies and biosimilars is also expected to drive the market. Strategic initiatives undertaken by leading companies, such as collaborations and agreements, are also expected to boost the development of new treatment modalities. The key players in the market, such as Amgen, Novartis, and Takeda, are investing in clinical trials and new product launches to strengthen their position in the market. These factors are expected to have a positive impact on the purpura treatment market in the coming years.
Regional Market Size
Regional Deep Dive
The purpura treatment market is characterized by the growing awareness of blood disorders and the advancement of treatment options across the regions. North America has a robust health care system and a significant investment in research and development. Europe has a strong regulatory framework that supports the development of new therapies. The Asia-Pacific region has an increasing access to health care, with a growing number of patients suffering from purpura, and therefore a growing demand for effective treatments. The Middle East and Africa have unique challenges such as limited access to health care, but they are gradually improving through international collaboration. Latin America is experiencing a high rate of awareness and diagnosis, which is driving the market growth.
Europe
- The European Medicines Agency (EMA) has streamlined the approval process for new treatments, encouraging pharmaceutical companies to bring novel therapies to market more quickly.
- Organizations such as the European Hematology Association are actively promoting awareness and education about purpura, which is expected to lead to earlier diagnosis and treatment.
Asia Pacific
- Countries like Japan and Australia are implementing national health programs aimed at improving the diagnosis and treatment of bleeding disorders, including purpura.
- The rise of telemedicine in the region is facilitating better access to specialists for patients with purpura, which is likely to enhance treatment adherence and outcomes.
Latin America
- The introduction of new health policies aimed at increasing access to healthcare services is expected to improve diagnosis rates of purpura in countries like Brazil and Mexico.
- Local pharmaceutical companies are beginning to invest in research and development for purpura treatments, which could lead to more affordable options for patients in the region.
North America
- The FDA has recently approved new biologic therapies for the treatment of immune thrombocytopenic purpura (ITP), enhancing treatment options for patients and driving market growth.
- Key players like Amgen and Novartis are investing heavily in clinical trials and research initiatives to develop innovative treatments, which is expected to significantly impact patient outcomes.
Middle East And Africa
- Collaborations between local governments and international health organizations are focusing on improving healthcare infrastructure, which is crucial for the management of purpura in underserved areas.
- Awareness campaigns led by organizations like the World Health Organization are helping to educate the public about bleeding disorders, potentially increasing the number of diagnosed cases.
Did You Know?
“Approximately 1 in 10,000 people are affected by immune thrombocytopenic purpura (ITP), a common form of purpura, highlighting the need for effective treatment options.” — National Organization for Rare Disorders (NORD)
Segmental Market Size
PURPURAE TREATMENT MARKET - The market for the treatment of purpura is currently undergoing a stable growth. The increase in the prevalence of blood diseases and the need for effective therapy for the symptoms and underlying causes are the main driving forces. The support of regulatory authorities for new therapies is also an important driver of market dynamics, which encourages pharmaceutical companies to invest in R & D. The market for the treatment of purpura is currently in the mature phase of development, with Amgen and Novartis leading the way in the development of targeted therapies. The main application areas are the treatment of immune thrombocytopenic purpura (ITP) and other related conditions, where biosimilars and corticosteroids are the mainstays of treatment. The development of the treatment of thrombopenia, with the focus on individual medicine and the integration of telemedical services, will also contribute to the growth of this market. Gene therapy and monoclonal antibodies are determining the development of purpura therapies and offer new ways to treat the disease.
Future Outlook
The market for purpura is projected to show significant growth from 2023 to 2032, growing from $1.86 billion to $3.1 billion at a CAGR of 7.3%. This growth is driven by an increasing occurrence of purpura-related conditions, increased awareness of treatment options, and the development of medical technology. As the patient-centric approach becomes more popular, the demand for effective and new drugs will increase, and the market penetration and usage rates will rise. The development of biological drugs and targeted therapies will provide more effective and more personalized treatment for purpura patients. The support for the policy environment and the increase in research and development funds for hemotology will also accelerate the development and commercialization of new drugs. Telemedicine and digital health solutions will further improve the treatment and monitoring of patients, and contribute to the overall market growth. The purpura industry will continue to change and evolve. Stakeholders need to be flexible to seize opportunities and meet the needs of purpura patients.
Purpura treatment Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









