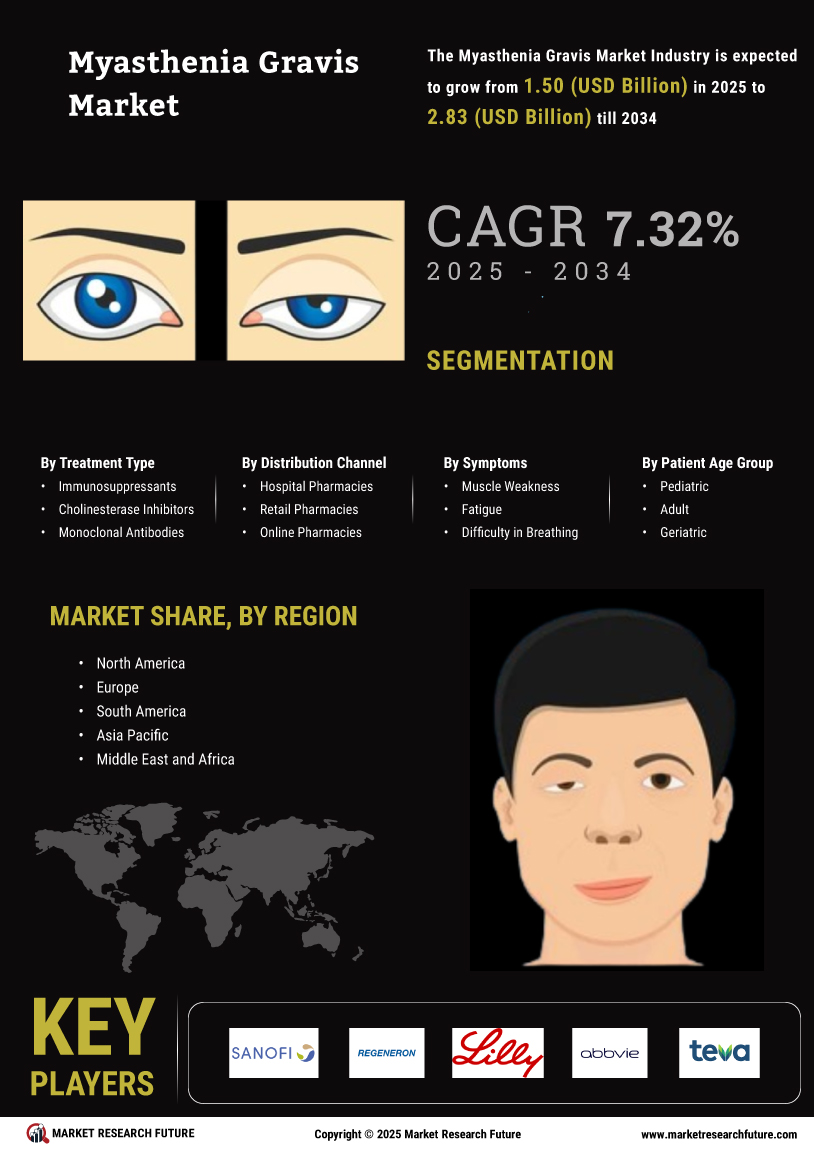
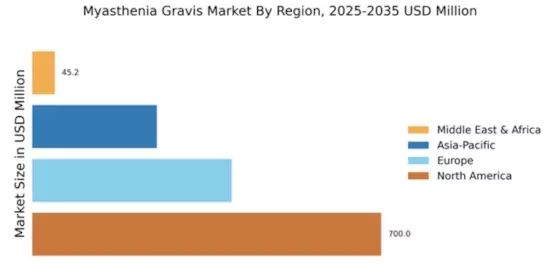
North America : Market Leader in Myasthenia Gravis
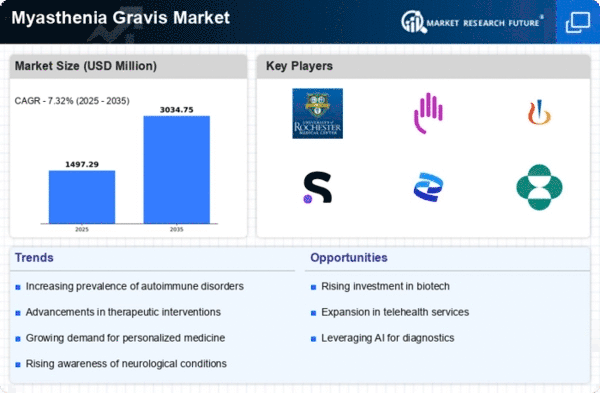
North America is poised to maintain its leadership in the Myasthenia Gravis market, holding a significant market share of $700.0M in 2024. The region's growth is driven by increasing prevalence rates, advancements in treatment options, and supportive regulatory frameworks. The FDA's expedited approval processes for innovative therapies further catalyze market expansion, meeting the rising demand for effective treatments. The competitive landscape in North America is robust, featuring key players such as Roche, Bristol-Myers Squibb, and Pfizer. These companies are actively engaged in research and development, focusing on novel therapies and personalized medicine. The presence of established healthcare infrastructure and investment in clinical trials enhances the region's attractiveness for market players, ensuring continued growth and innovation in the Myasthenia Gravis sector.
Europe : Emerging Market with Growth Potential
Europe is witnessing a growing Myasthenia Gravis market, valued at $400.0M in 2024. The region benefits from increasing awareness of the disease, improved diagnostic capabilities, and a rise in healthcare expenditure. Regulatory bodies are also playing a crucial role in facilitating access to new treatments, which is expected to drive market growth in the coming years. Leading countries such as Germany, France, and the UK are at the forefront of this market, supported by a strong presence of pharmaceutical companies like Novartis and Sanofi. The competitive landscape is characterized by strategic collaborations and partnerships aimed at enhancing treatment options. As the market evolves, the focus on patient-centric approaches and innovative therapies will be key to capturing market share.
Asia-Pacific : Emerging Powerhouse in Healthcare
The Asia-Pacific region is emerging as a significant player in the Myasthenia Gravis market, with a market size of $250.0M in 2024. Factors such as increasing healthcare investments, rising awareness of neurological disorders, and a growing patient population are driving demand. Additionally, supportive government initiatives aimed at improving healthcare access are expected to further boost market growth in this region. Countries like Japan, China, and Australia are leading the charge, with a growing number of clinical trials and research activities. Key players such as Eisai and Amgen are actively involved in developing innovative therapies tailored to the needs of the region. The competitive landscape is evolving, with a focus on collaboration between local and international firms to enhance treatment availability and patient outcomes.
Middle East and Africa : Developing Market with Challenges
The Middle East and Africa (MEA) region is gradually developing its Myasthenia Gravis market, currently valued at $45.16M in 2024. The growth is hindered by challenges such as limited healthcare infrastructure and varying levels of disease awareness. However, increasing investments in healthcare and initiatives to improve access to treatments are expected to drive market growth in the coming years. Countries like South Africa and the UAE are leading the market, with efforts to enhance healthcare services and patient education. The presence of global players is also increasing, as companies seek to tap into this emerging market. Collaborative efforts between governments and private sectors are essential to address the challenges and improve treatment accessibility for patients in the region.