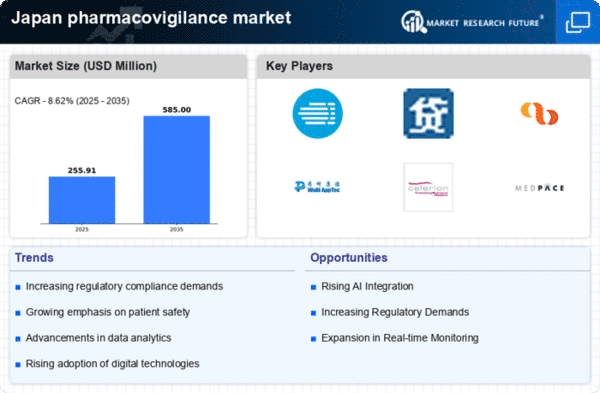
Increasing Regulatory Scrutiny
The pharmacovigilance market in Japan is experiencing heightened regulatory scrutiny, which is driving the demand for robust safety monitoring systems. Regulatory bodies, such as the Pharmaceuticals and Medical Devices Agency (PMDA), are enforcing stricter guidelines for drug safety reporting. This has led to an increased need for companies to invest in comprehensive pharmacovigilance systems to ensure compliance. As a result, the market is projected to grow at a CAGR of approximately 8% over the next few years. Companies are now prioritizing the implementation of advanced reporting tools and data analytics to meet these regulatory requirements, thereby enhancing the overall safety profile of pharmaceuticals in the market.
Expansion of Biopharmaceuticals
The rapid expansion of biopharmaceuticals is significantly influencing the pharmacovigilance market in Japan. As the biopharmaceutical sector continues to grow, the complexity of monitoring these products increases. Biologics often have unique safety profiles that require specialized pharmacovigilance strategies. Consequently, pharmaceutical companies are investing in tailored monitoring systems to address the specific challenges associated with biopharmaceuticals. This trend is expected to drive market growth, as organizations seek to ensure compliance with safety regulations while effectively managing the risks associated with these innovative therapies.
Technological Integration in Healthcare
The integration of advanced technologies into healthcare practices is transforming the pharmacovigilance market in Japan. Innovations such as artificial intelligence (AI) and machine learning are being utilized to enhance data analysis and reporting processes. These technologies enable faster identification of safety signals and improve the efficiency of pharmacovigilance operations. As a result, organizations are increasingly adopting these solutions to streamline their safety monitoring efforts. The market is projected to witness a growth rate of around 7% annually, driven by the demand for more efficient and accurate pharmacovigilance systems that leverage technological advancements.
Rising Incidence of Adverse Drug Reactions
The growing awareness of adverse drug reactions (ADRs) is significantly impacting the pharmacovigilance market in Japan. With an increasing number of medications being prescribed, the incidence of ADRs has also risen, prompting healthcare providers to prioritize patient safety. Reports indicate that ADRs account for nearly 10% of hospital admissions in Japan, underscoring the need for effective monitoring systems. This trend is likely to drive investments in pharmacovigilance solutions, as healthcare organizations seek to mitigate risks associated with drug therapies. Consequently, the market is expected to expand as stakeholders recognize the importance of proactive safety measures in improving patient outcomes.
Growing Demand for Patient-Centric Approaches
There is a noticeable shift towards patient-centric approaches in the pharmacovigilance market in Japan. Stakeholders are recognizing the importance of incorporating patient feedback into safety monitoring processes. This trend is leading to the development of more transparent reporting systems that prioritize patient experiences and outcomes. As patients become more engaged in their healthcare, the demand for pharmacovigilance solutions that facilitate direct communication and feedback is likely to increase. This evolution may result in a more responsive pharmacovigilance framework, ultimately enhancing drug safety and efficacy in the market.