Expansion of Healthcare Facilities
The expansion of healthcare facilities across Japan is anticipated to positively influence the heparin market. With an increasing number of hospitals and clinics, the accessibility of heparin treatments is likely to improve. This expansion is driven by the need to accommodate the aging population and the rising prevalence of chronic diseases. According to recent data, the number of healthcare facilities in Japan has increased by approximately 5% over the past few years. This growth may lead to higher demand for anticoagulants, including heparin, as healthcare providers seek to offer comprehensive treatment options. Consequently, the heparin market could experience significant growth as more patients gain access to essential therapies.
Advancements in Pharmaceutical Research
Advancements in pharmaceutical research and development are expected to play a pivotal role in shaping the heparin market in Japan. Innovative formulations and delivery methods for heparin are being explored, which may enhance its efficacy and safety profile. Research institutions and pharmaceutical companies are increasingly focusing on developing new heparin derivatives and formulations that could cater to specific patient needs. This trend indicates a potential for growth in the heparin market, as improved products may attract more healthcare providers and patients. The ongoing investment in research suggests that the heparin market could witness a transformation in product offerings, leading to increased competition and market expansion.
Rising Awareness of Preventive Healthcare
There is a growing awareness of preventive healthcare measures among the Japanese population, which is likely to impact the heparin market positively. As individuals become more informed about the risks associated with thromboembolic disorders, the demand for preventive anticoagulant therapies, such as heparin, may increase. Educational campaigns and healthcare initiatives aimed at promoting awareness of cardiovascular health are becoming more prevalent. This shift in consumer behavior suggests that patients are more inclined to seek preventive treatments, thereby driving the heparin market. The potential for increased adoption of heparin as a preventive measure could lead to a notable rise in market demand.
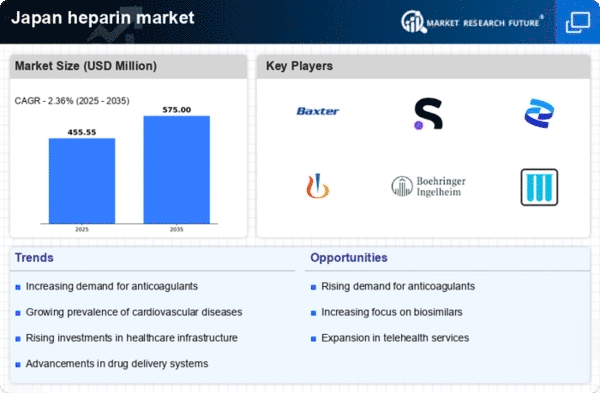
Increasing Prevalence of Cardiovascular Diseases
The rising incidence of cardiovascular diseases in Japan is a crucial driver for the heparin market. As the population ages, the demand for anticoagulants, including heparin, is expected to surge. Reports indicate that cardiovascular diseases account for a significant portion of mortality in Japan, prompting healthcare providers to seek effective treatment options. The heparin market is likely to benefit from this trend, as healthcare systems prioritize anticoagulant therapies to manage these conditions. Furthermore, the Japanese government has been investing in healthcare infrastructure, which may enhance access to heparin treatments. This growing focus on cardiovascular health suggests a robust future for the heparin market in Japan.
Government Initiatives to Improve Healthcare Access
Government initiatives aimed at improving healthcare access in Japan are likely to have a significant impact on the heparin market. Policies that promote the availability of essential medications, including anticoagulants, are being implemented to ensure that patients receive timely and effective treatments. The Japanese government has been actively working to reduce healthcare disparities, which may enhance access to heparin therapies for underserved populations. This focus on equitable healthcare access suggests that the heparin market could experience growth as more individuals gain access to necessary treatments. The potential for increased patient enrollment in anticoagulant therapies may drive demand for heparin in the coming years.