Rising Prevalence of Chronic Diseases
The increasing incidence of chronic diseases in Italy is a pivotal driver for the italy molecular diagnostics transplant market. Conditions such as diabetes, cardiovascular diseases, and various cancers necessitate advanced diagnostic tools for effective management and treatment. According to the Italian Ministry of Health, chronic diseases account for approximately 80% of healthcare expenditures in the country. This trend underscores the need for molecular diagnostics, which can provide precise information about disease progression and treatment efficacy. As healthcare providers seek to enhance patient outcomes, the demand for molecular diagnostics in transplant settings is likely to grow, facilitating personalized medicine approaches that are tailored to individual patient profiles.
Growing Awareness of Personalized Medicine
The shift towards personalized medicine is reshaping the italy molecular diagnostics transplant market. Patients and healthcare providers are increasingly recognizing the value of tailored treatment plans that consider individual genetic profiles. This trend is supported by educational initiatives aimed at raising awareness about the benefits of molecular diagnostics in transplant settings. As patients become more informed, they are likely to demand more precise diagnostic tests that can guide treatment decisions. Furthermore, healthcare providers are adapting their practices to incorporate molecular diagnostics, which can lead to improved patient outcomes and reduced healthcare costs. This growing emphasis on personalized medicine is expected to drive the demand for molecular diagnostics in Italy.
Regulatory Framework Supporting Innovation
Italy's regulatory environment plays a significant role in fostering innovation within the italy molecular diagnostics transplant market. The Italian Medicines Agency (AIFA) has established guidelines that encourage the development and approval of new diagnostic tests. These regulations aim to streamline the process for bringing innovative molecular diagnostics to market, ensuring that patients have access to the latest technologies. Additionally, the European Union's Medical Device Regulation (MDR) provides a framework that supports the introduction of advanced diagnostic tools while maintaining high safety and efficacy standards. This supportive regulatory landscape is likely to stimulate investment in research and development, further propelling the growth of the molecular diagnostics sector in Italy.
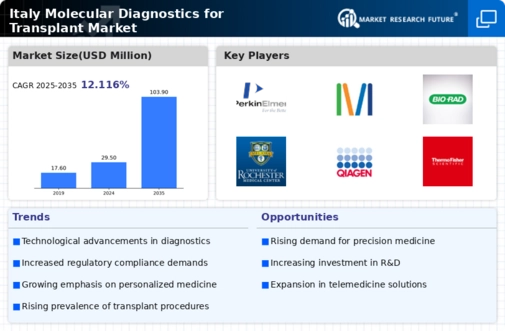
Technological Advancements in Diagnostic Tools
Technological innovations are transforming the landscape of the italy molecular diagnostics transplant market. The integration of next-generation sequencing (NGS) and polymerase chain reaction (PCR) technologies has significantly improved the accuracy and speed of diagnostic tests. These advancements enable healthcare professionals to identify genetic markers associated with transplant compatibility and rejection, thereby enhancing patient management. The Italian government has been actively promoting research and development in biotechnology, which has led to the emergence of several startups focused on molecular diagnostics. This dynamic environment fosters competition and innovation, ultimately benefiting patients and healthcare providers alike.
Increased Investment in Healthcare Infrastructure
Italy's commitment to enhancing its healthcare infrastructure is a crucial driver for the italy molecular diagnostics transplant market. The government has allocated substantial funding towards modernizing healthcare facilities and integrating advanced diagnostic technologies. Recent reports indicate that Italy's healthcare expenditure is projected to reach 8.5% of GDP by 2026, reflecting a strong focus on improving healthcare services. This investment is likely to facilitate the adoption of molecular diagnostics in transplant procedures, as hospitals and clinics upgrade their capabilities to provide state-of-the-art care. Enhanced infrastructure not only supports the implementation of new technologies but also ensures that healthcare professionals are adequately trained to utilize these advancements effectively.