Rising Prevalence of Chronic Diseases
The increasing incidence of chronic diseases such as diabetes, cardiovascular disorders, and cancer is a primary driver of the In Vitro Diagnostics Market. As healthcare systems strive to manage these conditions effectively, the demand for diagnostic tests that can provide timely and accurate results is surging. For instance, the World Health Organization has reported that chronic diseases account for approximately 70% of all deaths worldwide. This trend necessitates the development and deployment of advanced diagnostic tools, thereby propelling the In Vitro Diagnostics Market forward. Furthermore, the growing aging population, which is more susceptible to these diseases, is likely to further amplify the demand for in vitro diagnostic solutions.
Growing Awareness of Personalized Medicine
The rising awareness and adoption of personalized medicine are shaping the In Vitro Diagnostics Market. Personalized medicine, which tailors treatment based on individual patient characteristics, relies heavily on accurate diagnostic testing. As healthcare providers increasingly utilize genetic and biomarker testing to inform treatment decisions, the demand for in vitro diagnostics is expected to grow. Market analysis indicates that the personalized medicine market is projected to reach USD 2 trillion by 2025, which will likely drive the In Vitro Diagnostics Market as well. This trend underscores the importance of precision diagnostics in improving patient outcomes and optimizing therapeutic strategies.
Increasing Demand for Preventive Healthcare
The growing emphasis on preventive healthcare is significantly influencing the In Vitro Diagnostics Market. As healthcare providers and patients alike recognize the importance of early detection and prevention of diseases, the demand for diagnostic tests is expected to rise. Preventive diagnostics can lead to timely interventions, reducing the overall burden on healthcare systems. Market data suggests that the preventive healthcare market is anticipated to grow at a rate of 8% annually, which will likely bolster the In Vitro Diagnostics Market. This shift towards preventive measures is fostering a culture of health awareness, thereby increasing the utilization of diagnostic tests across various demographics.
Regulatory Support and Reimbursement Policies
Supportive regulatory frameworks and favorable reimbursement policies are crucial drivers of the In Vitro Diagnostics Market. Governments and regulatory bodies are increasingly recognizing the value of in vitro diagnostics in enhancing patient care and reducing healthcare costs. For instance, streamlined approval processes for new diagnostic tests can expedite their entry into the market, fostering innovation. Additionally, reimbursement policies that cover a wide range of diagnostic tests encourage healthcare providers to adopt these technologies. This supportive environment is likely to enhance the growth prospects of the In Vitro Diagnostics Market, as more stakeholders become willing to invest in advanced diagnostic solutions.
Technological Innovations in Diagnostic Tools
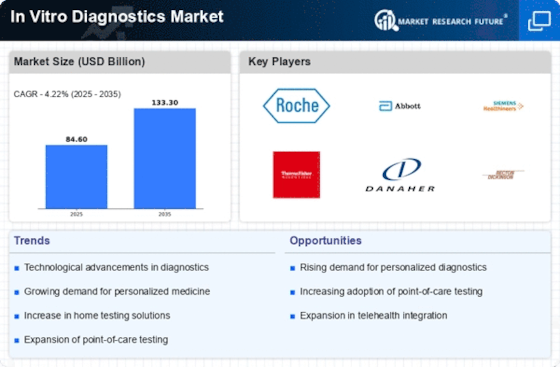
Technological advancements are revolutionizing the In Vitro Diagnostics Market, leading to the introduction of more sophisticated and efficient diagnostic tools. Innovations such as next-generation sequencing, microfluidics, and point-of-care testing devices are enhancing the accuracy and speed of diagnostic results. According to recent data, the market for molecular diagnostics alone is projected to reach USD 11 billion by 2026, reflecting a compound annual growth rate of over 10%. These innovations not only improve patient outcomes but also streamline laboratory workflows, making them essential in the evolving landscape of healthcare. As technology continues to advance, the In Vitro Diagnostics Market is expected to witness further growth driven by these cutting-edge solutions.


![In-Vitro Diagnostics [IVD] Market Regional Image In-Vitro Diagnostics [IVD] Market Regional Image](https://www.marketresearchfuture.com/uploads/reports/1165/in-vitro-diagnostics-market_reg_chart.webp)
![In-Vitro Diagnostics [IVD] Market key player In-Vitro Diagnostics [IVD] Market key player](https://www.marketresearchfuture.com/uploads/reports/1165/abbott-laboratories-us_keyplayer.webp)
![In-Vitro Diagnostics [IVD] Market key player In-Vitro Diagnostics [IVD] Market key player](https://www.marketresearchfuture.com/uploads/reports/1165/becton-dickinson-and-company-us_keyplayer.webp)
![In-Vitro Diagnostics [IVD] Market key player In-Vitro Diagnostics [IVD] Market key player](https://www.marketresearchfuture.com/uploads/reports/1165/danaher-corporation-us_keyplayer.webp)
![In-Vitro Diagnostics [IVD] Market key player In-Vitro Diagnostics [IVD] Market key player](https://www.marketresearchfuture.com/uploads/reports/1165/roche-diagnostics-ch_keyplayer.webp)
![In-Vitro Diagnostics [IVD] Market key player In-Vitro Diagnostics [IVD] Market key player](https://www.marketresearchfuture.com/uploads/reports/1165/siemens-healthineers-de_keyplayer.webp)
![In-Vitro Diagnostics [IVD] Market key player In-Vitro Diagnostics [IVD] Market key player](https://www.marketresearchfuture.com/uploads/reports/1165/thermo-fisher-scientific-us_keyplayer.webp)








