Market Growth Projections
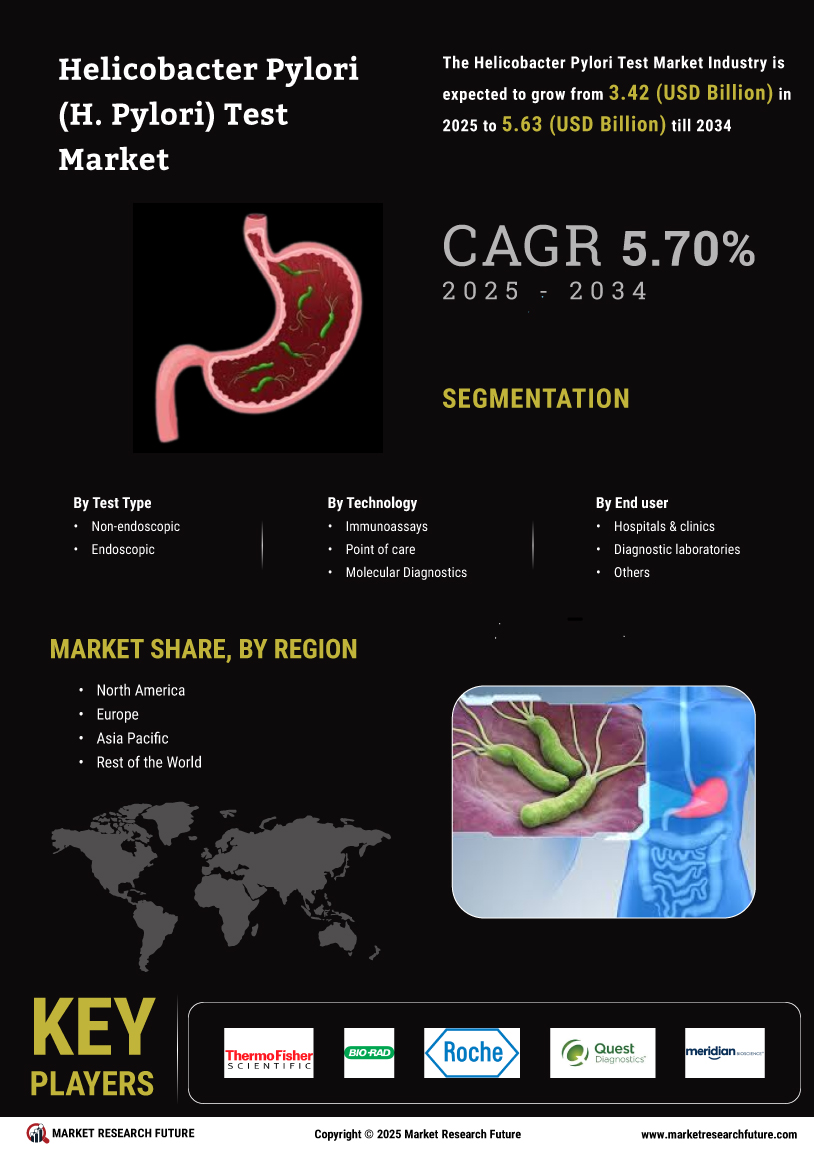
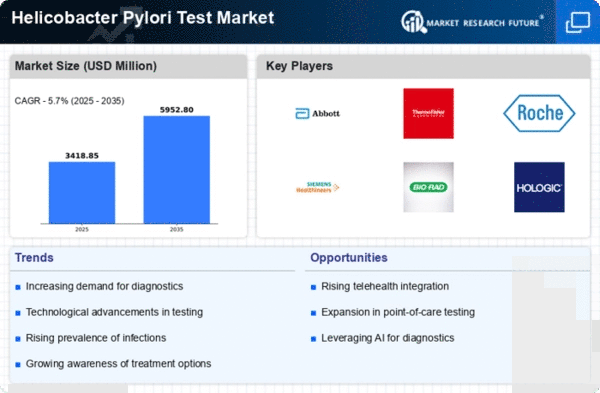
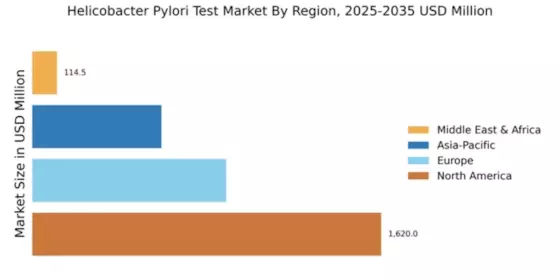
The Global Helicobacter Pylori Test Market Industry is projected to experience substantial growth over the next decade. With a market value of 3.23 USD Billion in 2024, it is anticipated to reach 5.95 USD Billion by 2035, reflecting a robust CAGR of 5.71% from 2025 to 2035. This growth trajectory indicates a rising demand for effective diagnostic solutions and highlights the increasing recognition of Helicobacter Pylori as a critical public health concern. The market's expansion is likely to be fueled by advancements in testing technologies, heightened awareness, and supportive regulatory frameworks.
Regulatory Support and Guidelines
Support from regulatory bodies and the establishment of clinical guidelines for Helicobacter Pylori testing bolster the Global Helicobacter Pylori Test Market Industry. Organizations such as the World Health Organization advocate for the testing and treatment of Helicobacter Pylori infections, influencing healthcare policies worldwide. This regulatory backing encourages healthcare providers to adopt testing protocols, thereby increasing market penetration. As guidelines evolve and emphasize the importance of early detection, the market is expected to experience sustained growth, driven by compliance with these recommendations.
Increase in Gastrointestinal Disorders
The rising prevalence of gastrointestinal disorders, such as gastritis and peptic ulcers, is a significant driver for the Global Helicobacter Pylori Test Market Industry. These conditions are often associated with Helicobacter Pylori infections, prompting healthcare professionals to recommend testing as a standard diagnostic procedure. As more individuals seek medical attention for gastrointestinal symptoms, the demand for reliable testing methods is expected to rise. This trend is likely to contribute to the market's growth, as healthcare systems prioritize effective diagnosis and management of these disorders.
Advancements in Diagnostic Technologies
Technological innovations in diagnostic methods are enhancing the accuracy and efficiency of Helicobacter Pylori testing. The Global Helicobacter Pylori Test Market Industry benefits from the introduction of non-invasive tests, such as breath tests and stool antigen tests, which are gaining traction among healthcare professionals. These advancements not only improve patient compliance but also reduce the burden on healthcare systems. As the market evolves, it is expected to grow at a CAGR of 5.71% from 2025 to 2035, potentially reaching 5.95 USD Billion by 2035, reflecting the increasing reliance on advanced diagnostic technologies.
Growing Awareness and Education Initiatives
Public health campaigns aimed at raising awareness about Helicobacter Pylori infections are crucial in driving the Global Helicobacter Pylori Test Market Industry. Educational initiatives by health organizations emphasize the importance of early detection and treatment of infections to prevent complications such as peptic ulcers and gastric cancer. This heightened awareness leads to increased testing and diagnosis rates, thereby expanding the market. As healthcare providers and patients become more informed about the risks associated with Helicobacter Pylori, the demand for testing solutions is likely to surge.
Rising Prevalence of Helicobacter Pylori Infections
The increasing incidence of Helicobacter Pylori infections globally drives the demand for testing solutions. According to health statistics, approximately 50% of the world's population is estimated to be infected with this bacterium, which is linked to various gastrointestinal disorders. The Global Helicobacter Pylori Test Market Industry is poised to grow significantly as awareness of these infections rises. With the market projected to reach 3.23 USD Billion in 2024, healthcare providers are increasingly adopting diagnostic tests to manage and treat affected individuals effectively.