Supportive Regulatory Frameworks
The organ on-chip market in Germany benefits from a supportive regulatory framework that encourages innovation and adoption of new technologies. Regulatory agencies are increasingly recognizing the potential of organ-on-chip systems in drug development and toxicity testing. In 2025, new guidelines are anticipated to be established, promoting the use of these technologies as alternatives to traditional testing methods. This regulatory support is likely to enhance the credibility and acceptance of organ-on-chip systems within the scientific community and industry. As a result, the organ on-chip market is expected to experience accelerated growth, as stakeholders align their research and development efforts with evolving regulatory standards.
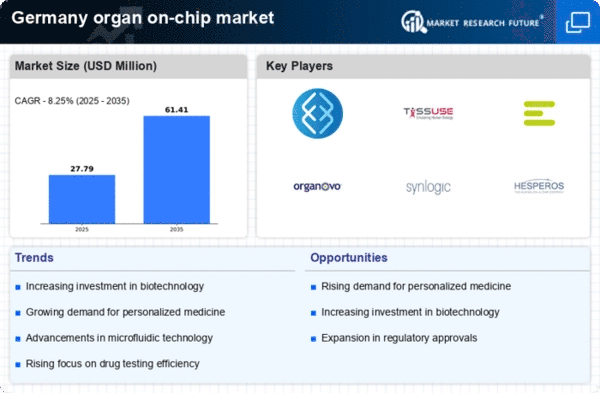
Rising Demand for Personalized Medicine
The organ on-chip market in Germany is experiencing a notable surge in demand driven by the increasing focus on personalized medicine. As healthcare shifts towards tailored treatments, the need for advanced models that accurately mimic human physiology becomes paramount. Organ-on-chip technologies offer the potential to create patient-specific models, enabling more effective drug testing and disease modeling. This trend is reflected in the market, which is projected to grow at a CAGR of approximately 25% from 2025 to 2030. The ability to simulate individual responses to therapies could revolutionize treatment protocols, thereby enhancing patient outcomes and reducing healthcare costs. Consequently, the organ on-chip market is poised for substantial growth as stakeholders recognize the value of personalized approaches in medical research and development.
Increased Investment in Biotech Research
Germany's commitment to advancing biotechnology research significantly influences the organ on-chip market. The government and private sectors are channeling substantial investments into innovative research initiatives, fostering an environment conducive to technological advancements. In 2025, funding for biotech research in Germany is expected to exceed €1 billion, with a considerable portion allocated to organ-on-chip technologies. This influx of capital not only accelerates the development of sophisticated models but also enhances collaboration between academic institutions and industry players. As a result, the organ on-chip market is likely to benefit from enhanced research capabilities, leading to the introduction of novel products and applications that address critical healthcare challenges.
Advancements in Microfabrication Techniques
The organ on-chip market in Germany is significantly influenced by advancements in microfabrication techniques. Innovations in materials science and engineering are enabling the creation of more complex and functional organ-on-chip systems. These advancements allow for the integration of multiple organ systems on a single chip, enhancing the predictive power of these models. As of 2025, the market is witnessing a shift towards more sophisticated designs that can replicate intricate biological processes. This evolution is likely to attract investment and interest from pharmaceutical companies seeking to streamline drug development processes. Consequently, the organ on-chip market is expected to thrive as these technologies become more accessible and effective in simulating human biology.
Growing Awareness of Ethical Testing Alternatives
The organ on-chip market in Germany is gaining traction due to the increasing awareness of ethical alternatives to traditional animal testing. Regulatory bodies and consumers alike are advocating for humane testing methods, prompting researchers to seek innovative solutions. Organ-on-chip technologies provide a viable alternative, allowing for in vitro testing that reduces reliance on animal models. This shift is reflected in the market dynamics, as the organ on-chip market is projected to expand by approximately 20% annually. The ethical implications of using organ-on-chip systems resonate with both researchers and the public, potentially leading to broader acceptance and integration of these technologies in drug development and toxicity testing.