Cost Containment Initiatives
In Germany, healthcare cost containment initiatives are significantly influencing the insulin biosimilars market. The government and health insurance providers are increasingly focused on reducing healthcare expenditures, particularly in the area of chronic disease management. This has led to a push for the adoption of biosimilars, which are generally more affordable than their reference biologics. The insulin biosimilars market is poised to grow as healthcare systems prioritize cost-effective treatment options. For instance, the introduction of biosimilars can lead to savings of up to 30% compared to original insulin products, making them an attractive choice for both providers and patients. As cost containment measures continue to evolve, the insulin biosimilars market is likely to see increased uptake and integration into standard treatment protocols.
Increasing Prevalence of Diabetes
The rising incidence of diabetes in Germany is a critical driver for the insulin biosimilars market. As of recent data, approximately 7.5 million individuals in Germany are diagnosed with diabetes, a figure that is projected to increase. This growing patient population necessitates the availability of affordable insulin options, thereby propelling the demand for biosimilars. The insulin biosimilars market is expected to benefit from this trend, as healthcare providers seek cost-effective alternatives to traditional insulin therapies. Furthermore, the increasing awareness of diabetes management and the importance of insulin therapy among patients and healthcare professionals further supports the growth of this market. As the prevalence of diabetes continues to rise, the insulin biosimilars market is likely to expand, providing essential treatment options for a larger segment of the population.
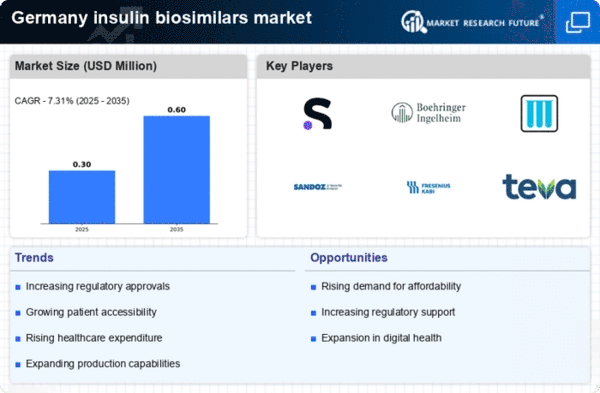
Regulatory Framework Enhancements
The regulatory landscape for biosimilars in Germany is evolving, which is likely to impact the insulin biosimilars market positively. Recent enhancements in the regulatory framework aim to streamline the approval process for biosimilars, making it easier for manufacturers to bring their products to market. This is particularly relevant for insulin biosimilars, as a more efficient regulatory pathway can lead to increased competition and lower prices for consumers. The German Medicines Agency has been actively working to establish clear guidelines for biosimilar approvals, which may encourage more companies to invest in the development of insulin biosimilars. As the regulatory environment becomes more favorable, the insulin biosimilars market is expected to expand, providing patients with greater access to affordable insulin therapies.
Advancements in Biologics Manufacturing
Technological advancements in biologics manufacturing are playing a pivotal role in shaping the insulin biosimilars market. Innovations in production processes, such as improved cell culture techniques and purification methods, have enhanced the efficiency and quality of biosimilar products. In Germany, these advancements are expected to lower production costs and increase the availability of high-quality insulin biosimilars. As manufacturers adopt these new technologies, the insulin biosimilars market may experience a surge in product offerings, catering to the diverse needs of patients. Moreover, the ability to produce biosimilars at a lower cost could lead to competitive pricing, further driving market growth. The ongoing evolution of manufacturing technologies is likely to be a key factor in the expansion of the insulin biosimilars market.
Growing Acceptance Among Healthcare Professionals
The increasing acceptance of biosimilars among healthcare professionals is a notable driver for the insulin biosimilars market. In Germany, physicians and endocrinologists are becoming more familiar with the efficacy and safety profiles of biosimilars, leading to a shift in prescribing practices. This growing confidence is crucial, as healthcare providers play a significant role in influencing patient treatment choices. As more clinical data supporting the use of biosimilars becomes available, the insulin biosimilars market is likely to benefit from enhanced trust and adoption. Additionally, educational initiatives aimed at healthcare professionals are fostering a better understanding of biosimilars, which may further facilitate their integration into diabetes management protocols. The evolving perception of biosimilars among practitioners is expected to positively impact the insulin biosimilars market.