Rising Demand for Biopharmaceuticals
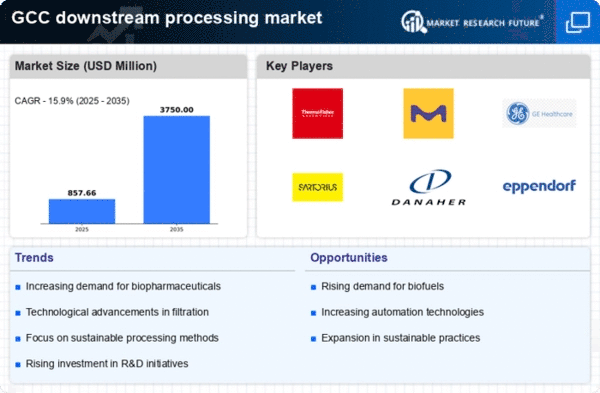
The increasing demand for biopharmaceuticals in the GCC region is a key driver for the downstream processing market. As healthcare systems evolve, there is a notable shift towards biologics, which are often more effective than traditional pharmaceuticals. This trend is reflected in the GCC's biopharmaceutical market, projected to reach approximately $10 billion by 2026. Consequently, downstream processing technologies are essential for the efficient purification and formulation of these products. The need for advanced filtration, chromatography, and other purification techniques is likely to grow, thereby enhancing the downstream processing market. Furthermore, the GCC's focus on improving healthcare infrastructure and regulatory frameworks supports this demand, indicating a robust future for the downstream processing market in the region.
Regulatory Support for Biotech Innovations
Regulatory support for biotechnology innovations is a crucial driver for the downstream processing market. The GCC governments are actively promoting policies that facilitate the development and commercialization of biotechnological products. This includes streamlined approval processes and incentives for research and development. For example, the UAE has established regulatory frameworks that encourage biotech startups, which in turn stimulates the downstream processing market. The region's commitment to fostering a conducive environment for biotech innovation is expected to enhance the demand for downstream processing solutions, as companies seek to comply with regulatory standards while ensuring product quality and safety.
Growing Focus on Quality Control and Compliance
The growing focus on quality control and compliance in the pharmaceutical and biotechnology sectors is driving the downstream processing market. As the GCC region enhances its regulatory landscape, companies are increasingly required to adhere to stringent quality standards. This necessitates the implementation of advanced downstream processing techniques that ensure product purity and safety. The market for quality control solutions is projected to grow at a CAGR of 8% through 2027, reflecting the rising importance of compliance in the industry. Consequently, the downstream processing market must adapt to these evolving standards, leading to increased investments in quality assurance technologies and practices.
Investment in Advanced Manufacturing Technologies
Investment in advanced manufacturing technologies is significantly influencing the downstream processing market. The GCC countries are increasingly adopting automation and digitalization in manufacturing processes, which enhances efficiency and reduces operational costs. For instance, the integration of artificial intelligence and machine learning in downstream processing can optimize production workflows and improve yield. Reports suggest that the adoption of such technologies could lead to a reduction in processing times by up to 30%. This trend not only boosts productivity but also aligns with the GCC's vision of diversifying its economy away from oil dependency. As a result, the downstream processing market is likely to experience substantial growth driven by these technological advancements.
Emergence of Contract Manufacturing Organizations (CMOs)
The emergence of Contract Manufacturing Organizations (CMOs) in the GCC is reshaping the downstream processing market. As pharmaceutical companies seek to optimize costs and focus on core competencies, they increasingly rely on CMOs for their manufacturing needs. This trend is particularly pronounced in the biopharmaceutical sector, where CMOs provide specialized downstream processing services. The CMO market in the GCC is expected to grow significantly, driven by the rising demand for outsourced manufacturing solutions. This shift not only allows for greater flexibility and scalability but also enhances the overall efficiency of the downstream processing market, as companies can leverage the expertise of CMOs to meet their production requirements.