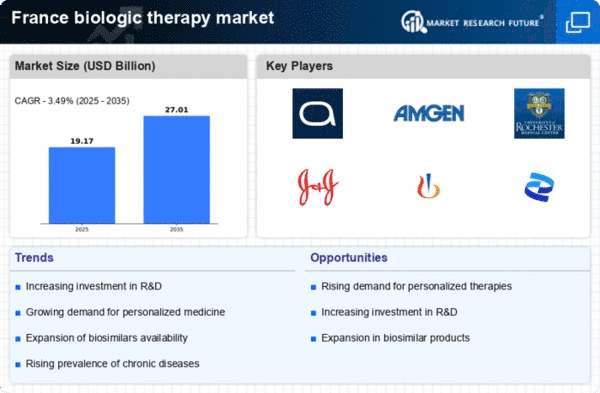
Advancements in Biologic Research
Ongoing advancements in biologic research are propelling the growth of the biologic therapy market. Innovations in biotechnology and genetic engineering are leading to the development of novel biologic agents that offer improved efficacy and safety profiles. In France, research institutions and pharmaceutical companies are investing heavily in R&D, with expenditures reaching approximately €3 billion annually. This investment is fostering a robust pipeline of new biologics, which are expected to enter the market in the coming years. The biologic therapy market is thus benefiting from a continuous influx of innovative products, which are likely to enhance treatment outcomes and patient satisfaction.
Supportive Reimbursement Policies
Supportive reimbursement policies in France are playing a crucial role in the growth of the biologic therapy market. The French healthcare system has established frameworks that facilitate the reimbursement of biologic treatments, making them more accessible to patients. Recent reforms have streamlined the approval process for new biologics, ensuring that effective therapies reach patients in a timely manner. As a result, the uptake of biologic therapies is expected to rise, with reimbursement rates for these treatments being favorable. The biologic therapy market is thus likely to benefit from these policies, as they enhance patient access and encourage healthcare providers to prescribe biologics.
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases in France is a pivotal driver for the biologic therapy market. Conditions such as rheumatoid arthritis, psoriasis, and various cancers are becoming more prevalent, necessitating advanced treatment options. According to recent health statistics, chronic diseases account for approximately 70% of all deaths in France, highlighting the urgent need for effective therapies. Biologic therapies, known for their targeted action and efficacy, are increasingly being adopted in clinical settings. This trend is likely to continue, as healthcare providers seek innovative solutions to manage these complex conditions. The biologic therapy market is thus positioned to expand significantly, driven by the demand for specialized treatments that address the unique needs of patients suffering from chronic ailments.
Rising Awareness and Education on Biologics
There is a growing awareness and education regarding biologic therapies among healthcare professionals and patients in France. Initiatives aimed at educating stakeholders about the benefits and applications of biologics are becoming more prevalent. This increased knowledge is likely to lead to higher adoption rates of biologic therapies in clinical practice. Surveys indicate that approximately 60% of healthcare providers in France are now familiar with the latest biologic treatments, compared to just 30% five years ago. The biologic therapy market is thus experiencing a shift, as informed patients and providers are more inclined to consider biologics as viable treatment options.
Growing Investment in Healthcare Infrastructure
The expansion of healthcare infrastructure in France is a significant driver for the biologic therapy market. The French government has been actively investing in healthcare facilities and services, with a budget allocation of over €200 billion for the healthcare sector in recent years. This investment is aimed at improving access to advanced therapies, including biologics. As hospitals and clinics upgrade their capabilities, the availability of biologic therapies is expected to increase, facilitating better patient access. The biologic therapy market stands to gain from this enhanced infrastructure, as more healthcare providers are equipped to administer these complex treatments.