

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
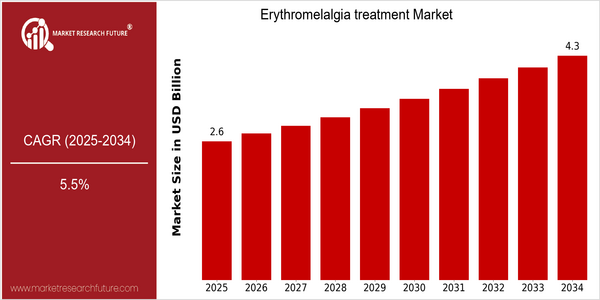
Market Size Snapshot
| Year | Value |
|---|---|
| 2025 | USD 2.64 Billion |
| 2034 | USD 4.27 Billion |
| CAGR (2025-2034) | 5.5 % |
Note – Market size depicts the revenue generated over the financial year
The erythromelalgia treatment market is expected to grow at a CAGR of 7.6% from 2017 to 2026, reaching a market size of USD 2.64 billion by 2026. This growth represents a CAGR of 5.5% during the forecast period. The increasing prevalence of erythromelalgia and the increasing awareness of the disease among health care professionals and patients are driving the demand for effective treatment options. Moreover, advancements in medical technology and pharmacological research are paving the way for the development of new therapies, thereby driving the market growth. The increasing occurrence of chronic pain disorders and the ongoing research on the underlying mechanisms of erythromelalgia are also expected to drive the market growth. Moreover, leading players such as Amgen, Pfizer, and Novartis are investing in the development of new therapies to address the unmet needs of patients. These initiatives are expected to enhance the treatment efficacy and improve patient outcomes, thereby fostering the market growth.
Regional Market Size
Regional Deep Dive
The Erythromelalgia treatment market is characterized by a rising awareness of the disease and an increasing demand for effective therapies across various regions. In North America, the market is driven by the advanced health care system, high prevalence of chronic pain disorders, and significant investment in research and development. Europe is characterized by a diverse regulatory framework that influences treatment options. Asia-Pacific is characterized by a rise in patient advocacy and awareness initiatives. Middle East and Africa are characterized by a lack of access to specialized care, but these issues are gradually being addressed through regional collaborations. Latin America is characterized by a growing interest from pharmaceutical companies in treating unmet medical needs in pain management.
Europe
- The European Medicines Agency (EMA) is currently reviewing several investigational drugs that target pain pathways, which could provide new treatment avenues for Erythromelalgia.
- Countries like Germany and the UK are implementing national pain management strategies that include Erythromelalgia, promoting better access to specialized care and treatment options.
Asia Pacific
- In countries like Japan and Australia, there is a growing trend towards personalized medicine, with research focusing on genetic factors influencing Erythromelalgia, which may lead to more effective treatments.
- Patient advocacy groups in India are pushing for better recognition of Erythromelalgia, which is expected to enhance research funding and treatment availability in the region.
Latin America
- Pharmaceutical companies are increasingly focusing on Latin America as a potential market for Erythromelalgia treatments, driven by a rising prevalence of chronic pain disorders.
- Government initiatives aimed at improving healthcare access are expected to facilitate better diagnosis and treatment options for Erythromelalgia patients in countries like Brazil and Mexico.
North America
- The U.S. Food and Drug Administration (FDA) has recently approved new treatment options for chronic pain conditions, which may indirectly benefit Erythromelalgia patients by expanding available therapies.
- Organizations like the Erythromelalgia Association are actively raising awareness and providing resources for patients, which is expected to lead to increased diagnosis and treatment rates.
Middle East And Africa
- The establishment of pain management clinics in countries like South Africa is improving access to care for Erythromelalgia patients, addressing a significant gap in treatment.
- Collaborations between local healthcare providers and international organizations are fostering knowledge exchange and training, which is crucial for improving diagnosis and treatment of rare conditions like Erythromelalgia.
Did You Know?
“Erythromelalgia is often misdiagnosed, with studies suggesting that up to 50% of patients may initially be treated for other conditions before receiving the correct diagnosis.” — Journal of Pain Research
Segmental Market Size
Among the most important treatments for erythromelalgia are the nebulizers, which are used for the treatment of the disease, and the use of the nebulizers is a means of bringing the patient's body to a state of relaxation. The use of the nebulizers is a means of treating the patient with a medicine that will suit the patient's needs. The use of the nebulizers is a means of bringing the patient's body to a state of relaxation. The use of the nebulizers is a means of bringing the patient's body to a state of relaxation. The stage of development of erythromelalgia treatment is now in its advanced stages, with such companies as Amgen and Pfizer leading the way in the development of the targeted therapies. The main purpose of the targeted therapies is pain management and symptom relief, and the most widely used therapies are the topical analgesics and the systemic drugs. The growing importance of the chronic pain management and the integration of telemedicine into the patient's consultation are trends that will accelerate the growth of the market. The development of the drug delivery systems and the use of the digital health technology are also shaping the evolution of the market, opening new avenues for effective treatment and patient engagement.
Future Outlook
The Erythromelalgia Market is projected to grow significantly from 2025 to 2034, with a CAGR of 5.5% from $2.64 billion to $ 4.27 billion. This growth is attributed to the rising prevalence of erythromelalgia, the rising awareness of the medical community and the rising number of treatment options. The penetration of this painful disease is expected to increase, reaching up to 15-20% of diagnosed cases by 2034, compared to 10-12 % in 2025. The development of new pharmacological therapies and the integration of telemedicine into the patient's care plan will reshape the therapeutic landscape. The ongoing research into the underlying mechanisms of erythromelalgia may lead to the development of targeted therapies with improved efficacy and safety profiles. Personalised medicine and patient-centred care will improve treatment adherence and outcomes, and will therefore increase the market's growth potential. The evolution of the health care system will bring new opportunities for the players in the industry to respond to the needs of patients suffering from this debilitating disease.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Market Size Value In 2022 | USD 2.25 Billion |
| Market Size Value In 2023 | USD 2.37 Billion |
| Growth Rate | 5.50% (2023-2032) |
Erythromelalgia Treatment Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









