China Healthcare Regulatory Affairs Outsourcing Market Overview
As per MRFR analysis, the China Healthcare Regulatory Affairs Outsourcing Market Size was estimated at 490 (USD Million) in 2023. The China Healthcare Regulatory Affairs Outsourcing Market Industry is expected to grow from 650(USD Million) in 2024 to 1,041 (USD Million) by 2035. The China Healthcare Regulatory Affairs Outsourcing Market CAGR (growth rate) is expected to be around 4.374% during the forecast period (2025 - 2035).
Key China Healthcare Regulatory Affairs Outsourcing Market Trends Highlighted
The China Healthcare Regulatory Affairs Outsourcing Market is experiencing significant growth, driven by several key market drivers. One of the primary drivers is the evolving regulatory environment in China, facilitated by initiatives from the National Medical Products Administration (NMPA) aimed at streamlining regulatory processes and ensuring compliance with international standards. This has increased demand for outsourcing regulatory affairs as companies seek expertise to navigate complex regulations efficiently. Moreover, the rising number of foreign investors entering the Chinese healthcare market is contributing to the need for specialized regulatory support, allowing companies to focus on their core competencies while ensuring adherence to local laws.This market is also experiencing an expansion of opportunities, particularly for companies that can provide innovative regulatory solutions that are specifically designed to address the distinctive characteristics of China's healthcare sector.
The demand for compliance services related to new medical devices, pharmaceuticals, and biologics is on the rise as the Chinese government prioritises the enhancement of healthcare quality and accessibility. Additionally, the increasing interest in telemedicine and digital health presents a niche area where regulatory outsourcing can capitalise on the demand for guidance on compliance in the swiftly advancing technology space. In recent years, there has been a greater emphasis on regulatory harmonisation as China attempts to align its standards with global practices. The outsourcing market's prospects are being enhanced by the adoption of more transparent approval processes and quicker timelines, which is attracting investments. Additionally, there is a greater emphasis on post-market surveillance, which compels companies to seek the assistance of specialists in order to adhere to the most recent regulations. The regulatory affairs outsourcing landscape is expected to undergo a period of transformation and opportunity in the market as China continues to expand as a healthcare centre in Asia.
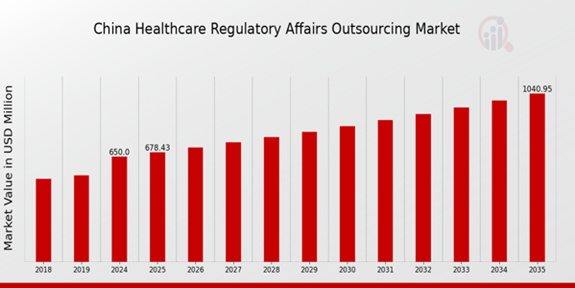
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
China Healthcare Regulatory Affairs Outsourcing Market Drivers
Increasing Regulatory Complexity in China
The regulatory landscape in China is becoming increasingly complex due to the evolving policies and guidelines proposed by the National Medical Products Administration (NMPA). As the Chinese government continues to implement reforms aimed at aligning with international standards, companies face the challenge of ensuring compliance with these new regulations. In 2020 alone, over 300 new regulations related to pharmaceuticals and medical devices were introduced, reflecting a trend towards stricter oversight.This complexity drives organizations to seek out specialized services in the China Healthcare Regulatory Affairs Outsourcing Market Industry, prompting them to partner with established regulatory affairs organizations like Parexel and Covance, which have expertise in navigating these substantial regulatory changes. Hence, increased demand for outsourcing regulatory affairs functions is expected to facilitate market growth in China.
Growth in the Pharmaceutical Sector
The pharmaceutical industry in China is witnessing rapid expansion, with a projected growth rate of 7.4% annually from 2020 to 2025, driven by demand for innovative therapeutics and an aging population. The China Food and Drug Administration reported over 200 new drug approvals in a single year, emphasizing the burgeoning market for pharmaceutical products. This growth necessitates effective regulatory strategies, which in turn increases demand for outsourced regulatory affairs services.Major pharmaceutical companies such as Sinopharm and Shanghai Pharmaceuticals are actively patenting new drugs, thereby putting additional pressure on regulatory services to meet compliance efficiently, thus propelling the China Healthcare Regulatory Affairs Outsourcing Market forward.
Rising Focus on Research and Development
Research and Development (R&D) investment in China’s healthcare sector is on the rise, reaching approximately 2.7% of the country's Gross Domestic Product (GDP) in recent years. The Chinese government has outlined a plan to boost R&D spending in health-related fields as part of its national strategy, targeting innovative drug development and medical technologies. This increased R&D investment translates to more clinical trials and regulatory submissions, subsequently increasing the demand for regulatory affairs outsourcing.Noteworthy organizations like WuXi AppTec have established extensive infrastructure to support these initiatives, further creating a tailored environment that supports the growth of the China Healthcare Regulatory Affairs Outsourcing Market.
Expanding Market for Medical Devices
The medical device market in China is expected to experience significant growth, with projections indicating a compound annual growth rate of approximately 10% from 2020 to 2025. Factors contributing to this growth include an aging population and increased healthcare spending. According to the Ministry of Health, the demand for advanced medical devices is spurred by the rising incidence of chronic diseases, leading to more hospitalizations and healthcare interventions.Companies like Mindray and Philips are major players in this space, frequently encountering new regulatory requirements that necessitate expert knowledge in compliance. As a result, the demand for regulatory affairs outsourcing services within the China Healthcare Regulatory Affairs Outsourcing Market has surged, fostering opportunities for growth in this segment of the industry.
China Healthcare Regulatory Affairs Outsourcing Market Segment Insights
Healthcare Regulatory Affairs Outsourcing Market Service Insights
The Service segment of the China Healthcare Regulatory Affairs Outsourcing Market has witnessed substantial growth, underpinned by an increasing demand for compliance with local regulations and the complexities involved in navigating the robust regulatory landscape. This sector is essential as it provides critical support for companies seeking to launch and market their healthcare products in China, a mammoth market characterized by a rapidly aging population and a growing emphasis on healthcare quality and safety. Regulatory Writing and Publishing play a significant role in ensuring that documentation complies with the guidelines set forth by regulatory agencies, thereby facilitating a smooth approval process. The Regulatory Submissions component of the segment is vital, as it involves the preparation and submission of essential documents required for regulatory approval, significantly impacting the speed of product entry into the market.
Clinical Trial Applications and Services Registrations represent another critical aspect, helping firms navigate the specific requirements for clinical trials, which are increasingly necessary in China's pharmaceutical environment. Additionally, Regulatory Consulting and Legal Representation are crucial as they assist companies in strategizing their market entry and compliance efforts, reducing the risk of regulatory pitfalls and ensuring alignment with both international standards and local regulations. Other Regulatory Affairs services also contribute to this segment, addressing various compliance issues that arise as the market landscape evolves. The overall trend in this segment showcases a robust demand driven by an increasing number of healthcare innovations and the necessity for regulatory assurance amidst growing consumer expectations and government scrutiny. The breadth of services offered allows for a comprehensive approach to meeting regulatory needs, thus positioning businesses for success in the competitive Chinese healthcare marketplace.
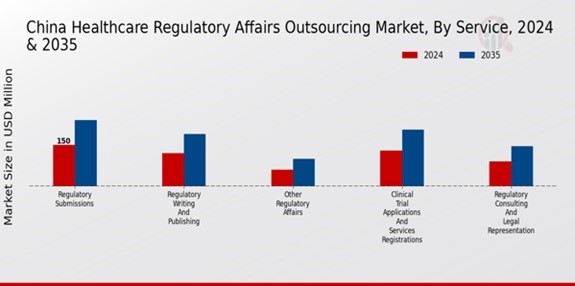
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review
Healthcare Regulatory Affairs Outsourcing Market End User Insights
The End User segment of the China Healthcare Regulatory Affairs Outsourcing Market plays a fundamental role in shaping the overall dynamics of the industry. Within this domain, various sectors significantly contribute to regulatory compliance efforts. Large Pharmaceutical Companies often seek comprehensive outsourcing solutions, leveraging expertise to navigate complex regulatory landscapes efficiently. Mid-Size Pharmaceutical Companies, on the other hand, benefit from outsourcing by accessing specialized knowledge without the heavy investments typical of larger firms.Biotechnology Companies are becoming increasingly important as they push innovation, requiring agile regulatory strategies to bring groundbreaking therapies to market.
Medical Device Companies also play a vital role, as the regulatory environment for devices demands rigorous compliance to ensure safety and efficacy. Additionally, Food and Beverage Companies are expanding their regulatory frameworks to align with health trends and consumer demands in China. The interplay between these segments represents a growing need for tailored regulatory services to address sector-specific challenges and enhance compliance efficiency.Overall, this segmentation reflects the diverse needs present in the marketplace, underscoring the importance of specialized knowledge and support in navigating regulatory processes effectively.
China Healthcare Regulatory Affairs Outsourcing Market Key Players and Competitive Insights
The China Healthcare Regulatory Affairs Outsourcing Market is undergoing significant transformations driven by regulatory changes, advancements in technology, and the increasing complexity of the healthcare landscape. As the demand for healthcare services continues to grow, the outsourcing of regulatory affairs has become a strategic approach for companies looking to navigate the stringent and evolving requirements set by local and international regulatory bodies. The competitive insights within this market reveal that companies are fostering collaborations, investing in innovation, and developing specialized services to enhance market adaptability and ensure compliance. Moreover, the growing trend of biopharmaceutical research and development is creating immense opportunities for regulatory affairs outsourcing, compelling established players and new entrants alike to refine their offerings and leverage their expertise in order to gain a competitive edge.
Medpace plays a crucial role in the China Healthcare Regulatory Affairs Outsourcing Market, leveraging its extensive knowledge and robust infrastructure to provide a wide spectrum of regulatory services. The company is well-established in the region, capitalizing on its deep understanding of local regulations and the healthcare ecosystem. Medpace's strengths lie in its comprehensive approach, offering integrated solutions that encompass regulatory strategy, submission management, and post-marketing compliance. This holistic service package empowers clients to expedite their product development processes while ensuring adherence to all regulatory requirements. Medpace's dedication to maintaining high standards of quality and operational efficiency further enhances its reputation in the market, positioning the company as a reliable partner for organizations seeking to navigate the complex regulatory landscape in China.
Veristat has also made substantial strides in the China Healthcare Regulatory Affairs Outsourcing Market, providing a diverse array of services aimed at supporting the life sciences sector. The company specializes in regulatory consulting, clinical trial management, and product registration services, catering to the unique needs of clients operating within China's dynamic market. Veristat's strengths are reflected in its commitment to delivering tailored solutions that align with regulatory expectations, thereby facilitating faster market entry for new products. The company has actively pursued strategic mergers and acquisitions to bolster its capabilities and expand its reach within China, further enhancing its operational presence. Veristat's focus on establishing strong partnerships with local stakeholders and its thorough understanding of the regulatory landscape place it in a prime position to offer invaluable support to companies looking to succeed in the competitive healthcare environment of China.
Key Companies in the China Healthcare Regulatory Affairs Outsourcing Market Include
- Charles River Laboratories
- Regulatory Affairs Professionals Society
- Pharmaceutical Product Development
China Healthcare Regulatory Affairs Outsourcing Market Industry Developments
Recent developments in the China Healthcare Regulatory Affairs Outsourcing Market have shown dynamic changes, particularly influenced by the growing demand for regulatory expertise in the fast-evolving healthcare sector. Companies like Medpace and WuXi AppTec have been expanding their services to meet increased regulatory requirements imposed by Chinese authorities. In terms of mergers and acquisitions, Celerion announced a strategic acquisition in July 2023 aimed at enhancing its operational capabilities in China, which underscores the trend of consolidation in the market. Syneos Health also announced an acquisition in March 2023 that broadened its regulatory services within the region.
The market has experienced significant growth, with a valuation surge attributed to the influx of foreign investment and the overall rise in clinical trials. In terms of major happenings, the Chinese government implemented new regulatory reforms in October 2022, which have streamlined the approval processes for new drugs and medical devices, further impacting the outsourcing landscape. Regulatory Affairs Professionals Society has reported an increase in the workforce specializing in regulatory affairs, signifying a trend toward professionalization in the industry. The overall market is poised for further growth, enhancing the competitive landscape among key players like Charles River Laboratories and Icon plc.
China Healthcare Regulatory Affairs Outsourcing Market Segmentation Insights
Healthcare Regulatory Affairs Outsourcing Market Service Outlook
- Regulatory Writing and Publishing
- Clinical Trial Applications and Services Registrations
- Regulatory Consulting and Legal Representation
Healthcare Regulatory Affairs Outsourcing Market End User Outlook
- Mid-Size Pharmaceutical Companies
- Large Pharmaceutical Companies
- Food and Beverage Companies
|
Market Size 2023
|
490.0(USD Million)
|
|
Market Size 2024
|
650.0(USD Million)
|
|
Market Size 2035
|
1041.0(USD Million)
|
|
Compound Annual Growth Rate (CAGR)
|
4.374% (2025 - 2035)
|
|
Report Coverage
|
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends
|
|
Base Year
|
2024
|
|
Market Forecast Period
|
2025 - 2035
|
|
Historical Data
|
2019 - 2024
|
|
Market Forecast Units
|
USD Million
|
|
Key Companies Profiled
|
Medpace, Veristat, WuXi AppTec, Celerion, Charles River Laboratories, Eurofins Scientific, Regulatory Affairs Professionals Society, Syneos Health, PAREXEL International, ClinChoice, CROMSOURCE, Sinovac Biotech, Icon plc, Pharmaceutical Product Development, PPD
|
|
Segments Covered
|
Service, End User
|
|
Key Market Opportunities
|
Increased demand for compliance services, Growing biopharmaceutical industry needs, Expanding clinical trials regulations support, Rising need for local expertise, Streamlined product approval processes
|
|
Key Market Dynamics
|
Regulatory compliance complexity, Rising demand for outsourcing, Increasing investment in healthcare, Emphasis on faster market access, Growing number of clinical trials
|
|
Countries Covered
|
China
|
Frequently Asked Questions (FAQ):
In 2024, the China Healthcare Regulatory Affairs Outsourcing Market is expected to be valued at 650.0 million USD.
By 2035, the market is projected to reach a value of 1,041.0 million USD.
The expected CAGR for the China Healthcare Regulatory Affairs Outsourcing Market from 2025 to 2035 is 4.374%.
Key players in the market include Medpace, Veristat, WuXi AppTec, and PAREXEL International among others.
Regulatory Writing and Publishing is valued at 120.0 million USD in 2024.
















