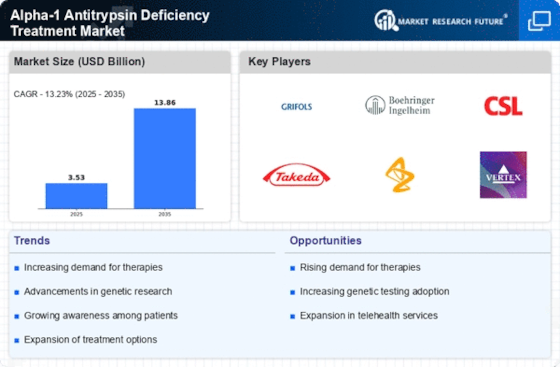
Regulatory Support for Orphan Drugs
Regulatory frameworks are increasingly supportive of the development of orphan drugs, which is a crucial factor for the Alpha-1 Antitrypsin Deficiency Treatment Market. Incentives such as tax breaks, extended market exclusivity, and expedited review processes are encouraging pharmaceutical companies to invest in treatments for rare diseases. This regulatory environment is particularly beneficial for conditions like Alpha-1 Antitrypsin Deficiency, where the patient population is limited. As a result, more companies are entering the market with innovative therapies, which could lead to a broader range of treatment options for patients. The favorable regulatory landscape is likely to stimulate competition and drive advancements in the development of effective therapies.
Rising Patient Advocacy and Support Groups
The emergence of patient advocacy organizations is playing a significant role in shaping the Alpha-1 Antitrypsin Deficiency Treatment Market. These groups are instrumental in raising awareness about the condition, providing education, and advocating for better treatment options. They often collaborate with healthcare providers and pharmaceutical companies to ensure that the needs of patients are met. The increasing visibility of these organizations is likely to lead to greater demand for therapies, as patients become more informed about their treatment options. Furthermore, these advocacy groups often engage in fundraising efforts, which can provide additional resources for research and development in the field. This growing support network is expected to enhance the overall landscape of treatment for Alpha-1 Antitrypsin Deficiency.
Growing Investment in Rare Disease Research
The Alpha-1 Antitrypsin Deficiency Treatment Market is benefiting from an increase in funding and investment directed towards rare disease research. Governments and private organizations are recognizing the need for targeted therapies for conditions like Alpha-1 Antitrypsin Deficiency, which have historically been underfunded. This shift in focus is leading to the development of new treatment options and improving the overall understanding of the disease. In recent years, funding for research initiatives has seen a notable increase, with millions allocated to studies aimed at discovering effective therapies. This influx of capital is likely to accelerate the pace of innovation within the market, ultimately benefiting patients and healthcare providers alike.
Technological Advancements in Treatment Modalities
Technological innovations are transforming the landscape of the Alpha-1 Antitrypsin Deficiency Treatment Market. Recent advancements in gene therapy and monoclonal antibodies have shown promise in providing more effective and targeted treatment options. For instance, the development of new therapies that can enhance the production of alpha-1 antitrypsin in the liver is gaining traction. These innovations not only improve patient outcomes but also attract significant investment from pharmaceutical companies. The market is witnessing a surge in clinical trials aimed at evaluating these novel therapies, which could potentially lead to breakthroughs in treatment. As these technologies mature, they are likely to reshape the treatment paradigm for Alpha-1 Antitrypsin Deficiency, creating new opportunities for market growth.
Increasing Prevalence of Alpha-1 Antitrypsin Deficiency
The rising incidence of Alpha-1 Antitrypsin Deficiency is a pivotal driver for the Alpha-1 Antitrypsin Deficiency Treatment Market. Recent estimates suggest that approximately 1 in 2,500 individuals are affected by this genetic disorder, leading to a growing patient population requiring treatment. As awareness of the condition increases, more individuals are being diagnosed, which in turn fuels demand for effective therapies. The increasing prevalence is particularly notable in certain demographics, such as individuals of European descent, where the deficiency is more common. This trend indicates a potential expansion in the market as healthcare providers seek to address the needs of this growing patient base, thereby driving innovation and investment in treatment options.