EMEA Sterility Testing Market Overview
The EMEA Sterility Testing Market is projected to reach USD 153.66 Million by 2030 at 13.20% CAGR during the forecast period 2022-2030.Sterilisation is any process that effectively eradicates any surface, equipment or article from the presence of viable microorganisms. Sterility testing is one of the important aspects of the healthcare sector which ensures the purity and safety of a product or a substance. Increasing prevalence of chronic diseases like cancer and HIV which can be easily transferred from the patient to the healthy adults through the use of the contaminated articles is one of the major drivers for the EMEA
sterility testing market growth. In 2015, according to the Centers for Disease Control and Prevention, 9,250 out of every 10,000 exposures to infected blood transfusion resulted in the transmission of the HIV. Similar, results were estimated by the use of infected needle during the parenteral intake of the drugs, 63 out of every 10,000 such exposures led to HIV. Moreover, expanding pharmaceutical and biotech industries, the rise in the approval of the drugs, growth in R&D investments in life science research along with rising healthcare and government support is expected to boost the market growth during the forecast period. However, precise regulatory and time-consuming approval processes along with the lack of skilled labor will restrain the EMEA sterility testing market growth during the forecast period.
Intended Audience
- Pharmaceutical companies
- Biotechnological institutes
- Research and Development (R&D) Companies
- Medical Research Laboratories
- Market Research and Consulting Service Providers
Figure 1:- EMEA sterility testing market share, by region 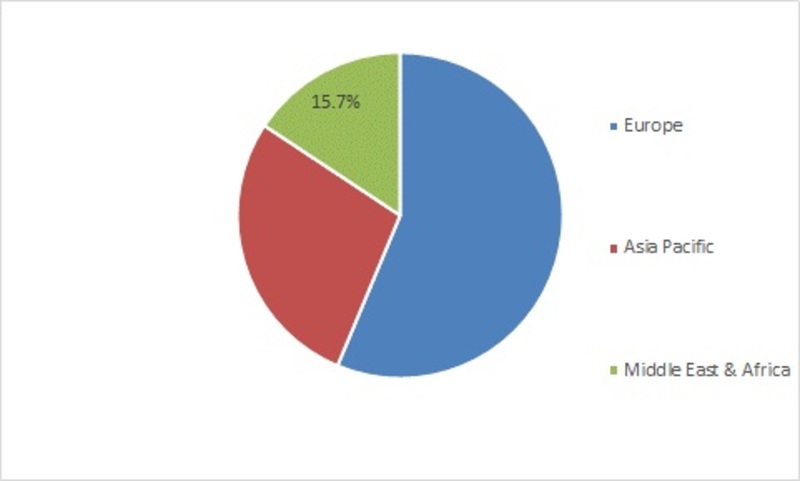
Sources: WHO, annual reports, press release, white paper, and company presentation
EMEA Sterility Testing Market Segment Insights
The EMEA sterility testing market is segmented on the basis of product, test, application and end users.
EMEA Sterility Testing Product Insights
The EMEA sterility testing market is segmented into kits & reagents, services, instruments, and others.
EMEA Sterility Testing Test Insights
The market is segmented into membrane filtration, direct inoculation sterility testing, and others. The membrane filtration segment is sub-segmented into membrane type which is further segmented into synthetic organic polymers and inorganic materials. The synthetic organic polymers, by membrane type, is sub-segmented into polysulfone, cellulose acetate, and others. The inorganic materials, by membrane type, is sub-segmented into ceramic membranes, metal membranes, others.
EMEA Sterility Testing Application Insights
The market is segmented into pharmaceutical industries, biotechnology industries, and others.
EMEA Sterility Testing End Users Insights
The market is segmented into hospitals & clinics, academic institutes, research organization, and others.
EMEA Sterility Testing Regional Insights
Europe is the largest sterility testing market. High healthcare expenditures, government support for research & development and huge patient population drives the European market. Moreover, growing biotechnology and pharmaceutical industries is boosting the market growth. Additionally, the presence of the developed economies within the region like the U.K and France is fuelling the market growth during the forecast period. Regionally Europe is divided into Western Europe and Eastern Europe. Western Europe leads the market within the region. On the other hand, there are huge opportunities for the market growth in Eastern Europe.
Asia Pacific is the fastest growing region in EMEA market due to the presence of a huge patient population and continuously developing economies like India and China which have a growing healthcare industry. According to the Indian Brand Equity Foundation, in 2017, the Indian healthcare sector is one of the fastest growing industries and is expected to advance at a CAGR of 22.8% and reach USD 280 billion by 2020. Additionally, favorable government policies like reduced excise and customs duty followed by the exemptions in service tax in India boosts the regional market growth.
The Middle East & Africa holds the least share in the EMEA sterility testing market due to the presence of poor economies in the African region owing to the low per capita income and stringent government policies within the region. A majority of the Middle East & Africa market is held by the Middle East due to huge healthcare expenditures and presence of developed economies like Kuwait, Saudi Arabia, Dubai, and Qatar.
Research Methodology 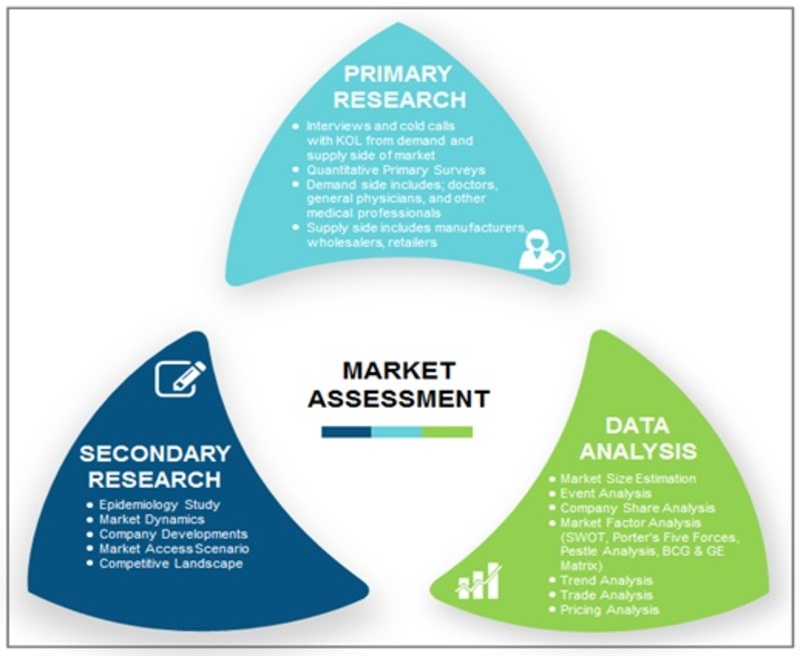
Sources: WHO, annual reports, press release, white paper, and company presentation
Key players for EMEA sterility testing market
Some of the major players in the EMEA sterility testing market are Merck KGaA (Germany), bioMérieux SA (France), SGS SA (Switzerland), Sartorius AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Toxikon, Inc. (U.S.), Charles River (U.S.), and others.
Europe Sterility Testing Market Highlights:





 Latest Sterility testing Companies Update
Latest Sterility testing Companies Update