- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
The pharmaceutical analytical testing outsourcing market is influenced by several key factors that shape its growth and dynamics. Firstly, the increasing complexity of drug development and regulatory requirements drives the demand for analytical testing services from pharmaceutical companies. As the pharmaceutical industry continues to innovate and develop new drug candidates, there is a growing need for comprehensive analytical testing to ensure the quality, safety, and efficacy of pharmaceutical products. Analytical testing plays a crucial role in drug development, from early-stage research and development (R&D) to manufacturing and regulatory approval. Pharmaceutical companies rely on outsourcing partners with specialized expertise and state-of-the-art facilities to conduct a wide range of analytical tests, including chemical characterization, stability testing, impurity profiling, and bioanalytical assays, to support product development and regulatory submissions.
Secondly, cost pressures and resource constraints incentivize pharmaceutical companies to outsource analytical testing activities to contract research organizations (CROs) and contract manufacturing organizations (CMOs) with specialized capabilities and infrastructure. Outsourcing analytical testing enables pharmaceutical companies to access advanced technologies, specialized expertise, and scalable resources without the need for significant upfront investments in laboratory equipment and personnel. By leveraging the expertise and infrastructure of outsourcing partners, pharmaceutical companies can streamline their operations, reduce time-to-market, and focus on core competencies such as drug discovery, formulation development, and commercialization, while maintaining high-quality analytical testing standards and compliance with regulatory requirements.
Another significant factor driving the pharmaceutical analytical testing outsourcing market is the globalization of drug development and manufacturing activities. Pharmaceutical companies increasingly rely on global networks of CROs and CMOs to support their analytical testing needs across different stages of the drug development lifecycle. Outsourcing analytical testing to international partners enables pharmaceutical companies to access a diverse talent pool, tap into regional expertise, and take advantage of cost efficiencies associated with offshore labor and lower operating costs. Additionally, the harmonization of regulatory standards and quality assurance requirements facilitates cross-border collaborations and enables seamless transfer of analytical testing data and results between global partners, supporting efficient and compliant drug development processes.
Moreover, the emergence of biopharmaceuticals, biosimilars, and personalized medicine therapies drives the demand for specialized analytical testing services tailored to the unique characteristics of biologic drugs and novel therapeutic modalities. Biopharmaceuticals, including monoclonal antibodies, recombinant proteins, and cell and gene therapies, require advanced analytical techniques such as mass spectrometry, chromatography, and protein characterization to assess product quality, purity, and potency. Outsourcing partners with expertise in biologics testing and advanced analytical methodologies play a critical role in supporting biopharmaceutical development and accelerating the commercialization of innovative therapies.
Furthermore, increasing regulatory scrutiny and quality standards in the pharmaceutical industry drive the need for robust analytical testing capabilities and compliance expertise. Regulatory agencies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) impose stringent requirements for analytical testing, data integrity, and Good Laboratory Practice (GLP) compliance. Outsourcing partners with a track record of regulatory compliance, accreditation, and audit readiness provide pharmaceutical companies with confidence in the accuracy, reliability, and regulatory acceptance of analytical testing data, facilitating regulatory submissions and approvals for new drug products.
Covered Aspects:
| Report Attribute/Metric | Details |
|---|---|
| Market Size Value In 2022 | USD 4.2 Billion |
| Market Size Value In 2023 | USD 4.6 Billion |
| Growth Rate | 8.70% (2023-2030) |
Pharmaceutical Analytical Testing Outsourcing Market Highlights:
Global Pharmaceutical Analytical Testing Outsourcing Market Overview
Pharmaceutical Analytical Testing Outsourcing Market Size was valued at USD 4.2 billion in 2022. The pharmaceutical analytical testing outsourcing market industry is projected to grow from USD 4.6 Billion in 2023 to USD 7.5 billion by 2030, exhibiting a compound annual growth rate (CAGR) of 8.70% during the forecast period (2023 - 2030). The market drivers for the expansion of the market are innovations in the pharmaceutical sector, a growing emphasis on regulation, safety, and quality, an increase in end users, and the cost advantages of outsourcing.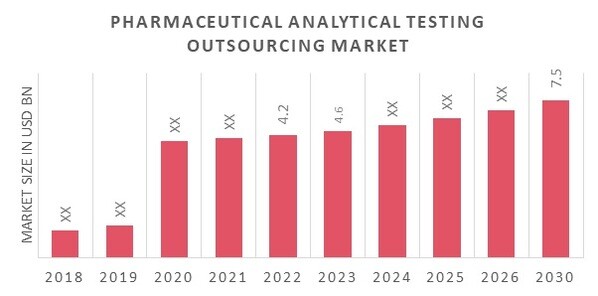 Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
The invention of pharmaceuticals resulted in a revolution in human health. If these pharmaceuticals' proper dosage and purity were provided, they would only serve their intended purpose. Identification of medication candidates for additional in-depth research requires the synthesis, characterization, and analysis of these molecules, also known as active pharmaceutical ingredients (APIs), and their examination to produce interim safety and therapeutic efficacy data. The "compound" that will become the drug molecule goes through a series of experiments and safety checks to demonstrate that it is absorbed into the bloodstream, distributed to the right site of action in the body, adequately metabolized, and demonstrates its non-toxicity. As a result, it can be deemed safe and effective. Since contaminants can occur at various times during production, shipping, and storage, rendering administration risky, these drugs must be identified and quantitated.
Clinical trial outsourcing possibilities are spreading more widely as industry laws change, incentives to save drug development costs rise, and demand for new medicines rises. Many companies have reevaluated their entire research strategy and typical staffing. Companies that contract out pharmaceutical analytical testing significantly contribute to clinical research since they have the expertise required for a clinical study to be properly developed. The market is primarily driven by pharmaceutical sector development, increased emphasis on regulation, safety, and quality, an increase in end users, and the cost advantages of outsourcing. The infrastructure required to enable various analytical testing types is absent in small and medium-sized pharma enterprises. Because it saves money, outsourcing these procedures is the best choice.
The need for medications has surged as a result of the COVID-19 pandemic. Pharmaceutical corporations, biotech firms, contract research organizations, and other end users relied heavily on worldwide pharmaceutical analytical testing outsourcing companies to meet their testing demands during this crisis. The factors influencing the growth of this market also include an increase in R&D spending, a focus on oversight, safety, and quality, pricing advantages of outsourcing, an increase in demand for analytical drugs as well as for biosimilars and biopharmaceutical products, and the accessibility of cutting-edge analytical testing methods like hyphenated techniques, kinetic methods of analysis, and electrochemical techniques, among others. Many chemical and instrumental procedures are utilized in drug assessment to ensure that medicines work as intended.
Pharmaceutical Analytical Testing Outsourcing Market Trends
- Increasing public awareness of pharmaceutical analytical testing outsourcing will boost market growth
The demand for external development knowledge and labour support from pharmaceutical and biotech businesses for their potential compounds has altered as pharmaceutical analytical testing outsourcing has consolidated at an accelerated rate. As industry regulations change, incentives to reduce drug development costs increase, and demand for novel treatments rises, clinical trial outsourcing options are becoming more and more common. Numerous businesses have reevaluated insourcing, including traditional staffing, and their entire research strategy. Companies that outsource pharmaceutical analytical testing are crucial contributors to clinical research because they possess the knowledge and skills necessary for a clinical study's proper development. Pharmaceutical, contract research organisations, and biotech firms gain from them because they relieve their load while ensuring trial quality and compliance with local, national, and international regulations. In addition, a number of pharmaceutical analytical testing outsourcing companies provide cutting-edge technology tools to enhance study process effectiveness, resulting in resource savings. Thus, this factor is driving the market CAGR for pharmaceutical analytical testing outsourcing.
Additionally, the market will experience significant expansion as a result of the increasing demand for biosimilars and biopharmaceuticals. For instance, it is predicted that the development of new biosimilars will result in consumer savings of up to USD 250 billion and increase access to biological therapies for an additional 1.2 million patients by 2025, according to a study titled "Expected Impact of Biosimilars on the Pharmaceutical Companies" that was published in the Iranian Journal of Medical Sciences in August 2021. This makes these items more accessible to patients with chronic diseases and gives them a more inexpensive alternative if they previously had to stop taking their prescriptions or rely on ineffective ones. A rise in the production of biosimilars will enhance the outsourcing of drug testing services, which will accelerate market expansion.
Furthermore, for businesses in the pharmaceutical and biotechnology industries, research and development is an investment in knowledge and technology. Drug development, research, process improvement, and innovation are areas where pharmaceutical corporations can invest a lot of time and money. Many industry players reported record sales, primarily as a result of strong growth in emerging markets like the USA, Japan, China, India, Germany, and the U.K., as well as rising government spending on pharmaceutical R&D in developing countries, advancements in the fields of drug discovery and clinical diagnostics, rising life science R&D expenditure, technological advancements, emerging applications, growing geriatric populations, rising prevalence of chronic diseases, a growing geriatric population, and a growing geriatric market. As a result, high-end research projects call for equipment with high throughput capacity and quality, further boosting the market for outsourcing pharmaceutical analytical testing. Thus, it is anticipated that this aspect will accelerate pharmaceutical analytical testing outsourcing market revenue globally.
Pharmaceutical Analytical Testing Outsourcing Market Segment Insights
Pharmaceutical Analytical Testing Outsourcing Product Insights
The Pharmaceutical Analytical Testing Outsourcing Market segmentation, based on product, includes Finished Products and Active Pharmaceutical ingredients. The active pharmaceutical ingredients segment held the majority share in 2022 in the Pharmaceutical Analytical Testing Outsourcing Market data. The essential components of medications are the active pharmaceutical ingredients, which are in charge of the patient's recovery. To eliminate contaminants, quality testing of pharmaceutical active components is required. Active pharmaceutical ingredients (APIs) can now be employed in several drug batches thanks to analytical testing, which has broadened their range of applications.
Pharmaceutical Analytical Testing Outsourcing Services Insights
The Pharmaceutical Analytical Testing Outsourcing Market segmentation, based on services, includes Bioanalytical testing, Stability testing, Method development & validation, and others. The stability testing segment dominated the market growth for pharmaceutical analytical testing outsourcing in 2022 and is projected to be the faster-growing segment during the forecast period, 2022-2030. Testing for stability determines whether a medicine can maintain its qualities over the course of its shelf life. One of the most crucial factors in the creation of new medications and formulations is the stability studies of pharmaceutical goods or medications. Moreover, the expansion of the industry is being fueled by the strategic actions made by the leading players, such as product launches, approvals, and alliances.
For instance, LGM Pharma began offering pharmaceutical researchers, manufacturers, and compounding pharmacies analytical testing and stability services in July 2021.
Pharmaceutical Analytical Testing Outsourcing End-User Insights
The Pharmaceutical Analytical Testing Outsourcing Market data, based on end-user, includes Biopharmaceutical Companies, Biotechnology Industry and Pharmaceutical. The pharmaceutical segment dominated the pharmaceutical analytical testing outsourcing market revenue for pharmaceutical analytical testing outsourcing in 2022 and is projected to be the faster-growing segment during the forecast period, 2022-2030. Many clinical trials are conducted on pharmaceutical goods with the goal of evaluating various aspects of a medicine. Several medium-sized and smaller pharmaceutical firms do not have the capability of internal testing facilities. These businesses can concentrate on core manufacturing by outsourcing pharmaceutical analytical testing.
Figure 1: Pharmaceutical Analytical Testing Outsourcing Market, by End-User, 2022 & 2030 (USD Billion)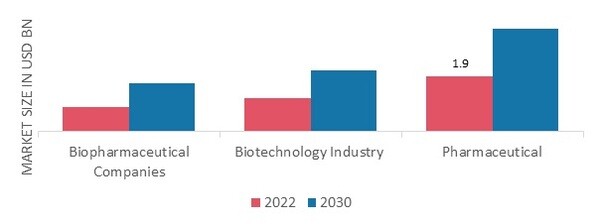 Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Pharmaceutical Analytical Testing Outsourcing Regional Insights
By region, the study provides the market insights for pharmaceutical analytical testing outsourcing into North America, Europe, Asia-Pacific and Rest of the World. North America pharmaceutical analytical testing outsourcing market accounted for USD 1.9 billion in 2022 with a share of around 45.80% and is expected to exhibit a significant CAGR growth during the study period. This is due to the area's dependable, complex, and advanced pharmaceutical production facilities. One of the main factors influencing the market is the quick expansion of pharmaceutical production capacity to fulfill the region's expanding demand for high-quality healthcare services.
Further, the major countries studied in the market report for pharmaceutical analytical testing outsourcing are: The U.S, Canada, Germany, France, UK, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil.
News
The Virginia Biotechnology Research Park's Advanced Pharmaceutical Development Center facilities in Richmond, US, were inaugurated by Phlow Corp. and US Pharmacopeia (USP). To enable them to work together on policies, best practices, and resources to aid in introducing advanced manufacturing techniques, the two partners' labs are "co-located" in one place. To create small molecule active pharmaceutical ingredients (APIs) plus key starting materials (KSMs), the organizations will use their facilities to deliver R&D services to the pharmaceutical sector and the US government. The partners claim they will use cutting-edge manufacturing methods to create high-quality, cost-effective medical products.
Figure 2: PHARMACEUTICAL ANALYTICAL TESTING OUTSOURCING MARKET SHARE BY REGION 2022 (%)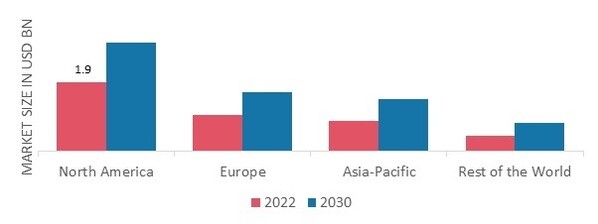 Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Europe pharmaceutical analytical testing outsourcing market accounts for the second-largest market share as one of the most cutting-edge manufacturing technologies now on the market, microbial manufacturing technology, has been enthusiastically embraced. In order to strengthen their domestic capabilities, several developing nations around the world are trying to collaborate with Western giants by providing industrial support services including clinical research, medicine development, and others. Further, the UK pharmaceutical analytical testing outsourcing market held the largest market share, and the Germany pharmaceutical analytical testing outsourcing market was the fastest growing market in the region.
Asia Pacific pharmaceutical analytical testing outsourcing market is expected to grow at the fastest CAGR from 2022 to 2030 due to strong expansion in the pharmaceutical industry, high levels of investment made in the area by major players, and the existence of sizable outsourcing hubs in China, the Philippines, and India. China is gaining favor as a delivery site for Japanese nearshore and onshore clients. Moreover, China pharmaceutical analytical testing outsourcing market held the largest market share, and the India pharmaceutical analytical testing outsourcing market was the fastest growing market in the region.
Pharmaceutical Analytical Testing Outsourcing Key Market Players & Competitive Insights
Major market players are spending a lot of money on R&D to increase their product lines, which will help the pharmaceutical analytical testing outsourcing market grow even more. Market participants are also taking a range of strategic initiatives to grow their worldwide footprint, with key market developments such as new product launches, contractual agreements, mergers and acquisitions, increased investments, and collaboration with other organizations. Competitors in the pharmaceutical analytical testing outsourcing industry must offer cost-effective items to expand and survive in an increasingly competitive and rising market environment.
One of the primary business strategies adopted by manufacturers in the global pharmaceutical analytical testing outsourcing industry to benefit clients and expand the market sector is to manufacture locally to reduce operating costs. In recent years, pharmaceutical analytical testing outsourcing industry has provided medicine with some of the most significant benefits. The pharmaceutical analytical testing outsourcing market major player such as Boston Analytical (New Hampshire), West Pharmaceutical Services, Inc. (U.S.), Exova Group PLC (U.K.), Source BioScience (U.K.), Pace Analytical Services, Inc. (U.S.), Merck KGaA (Germany), WuXi AppTec (US), Toxikon (US), Eurofins Scientific (Belgium), Intertek Group Plc (U.K.), Eurofins Scientific (Luxembourg), West Pharmaceutical Services Inc. (U.S.), Charles River Laboratories International, Inc (U.S.), SGS SA (U.K.), Pharmaceutical Product Development, LLC (U.S.), Source BioScience (U.K.) and WuXi AppTec (U.S.).
American company Thermo Fisher Scientific Inc. sells scientific equipment, reagents and supplies, and software services. Thermo Fisher was created in 2006 by the union of Thermo Electron and Fisher Scientific and is headquartered in Waltham, Massachusetts. Several reagent, consumable, instrument, and service suppliers have been purchased by Thermo Fisher Scientific. In December 2021, To increase its offering of laboratory services, clinical research, and analytical testing, among other things, Thermo Fisher fully purchased PPD.
For the CMC (Chemistry, Manufacturing, and Controls) factors involved in medication development and release testing, Boston Analytical specialists in offering high-quality analytical testing services. All necessary testing and related services, including analytical development, storage and stability testing, and microbial testing, can be performed in our cGMP, FDA accredited analytical testing laboratory. In January 2021, Boston Analytical, a Salem, New Hampshire-based cGMP-compliant analytical laboratory, is officially launching its second office in Morrisville, North Carolina. Sampling and testing services will be offered at this location.
Key Companies in the pharmaceutical analytical testing outsourcing market includes
- Boston Analytical (New Hampshire)
- West Pharmaceutical Services, Inc. (U.S.)
- Exova Group PLC (U.K.)
- Source BioScience (U.K.)
- Pace Analytical Services, Inc. (U.S.)
- Merck KGaA (Germany), WuXi AppTec (US)
- Toxikon (US), Eurofins Scientific (Belgium)
- Intertek Group Plc (U.K.)
- Eurofins Scientific (Luxembourg)
- West Pharmaceutical Services Inc. (U.S.)
- Charles River Laboratories International, Inc (U.S.)
- SGS SA (U.K.)
- Pharmaceutical Product Development, LLC (U.S.)
- Source BioScience (U.K.)
- WuXi AppTec (U.S.)
Pharmaceutical Analytical Testing Outsourcing Industry Developments
February 2022: The Center for Breakthrough Medicines (CBM) and BioAnalysis LLC (BIA) joined forces to form a strategic alliance that would give CBM's clients direct access to BIA's cutting-edge testing capabilities. These capabilities include the ability to characterise viral vectors using BIA's unique sedimentation velocity Approach to analytical ultracentrifugation, as well as other cutting-edge biophysical techniques and analytics.
December 2021: To improve its development testing capabilities for clients in the pharmaceutical, biotechnology, and medical device industries, LabCorp purchased Toxikon Corp. (TC).
Pharmaceutical Analytical Testing Outsourcing Market Segmentation
Pharmaceutical Analytical Testing Outsourcing Product Outlook (USD Billion, 2019-2030)
- Finished Products
- Active Pharmaceutical ingredients
Pharmaceutical Analytical Testing Outsourcing Services Outlook (USD Billion, 2019-2030)
- Bioanalytical Testing
- Stability Testing
- Method Development & Validation
- Others
Pharmaceutical Analytical Testing Outsourcing End User Outlook (USD Billion, 2019-2030)
- Biopharmaceutical Companies
- Biotechnology Industry
- Pharmaceutical
Pharmaceutical Analytical Testing Outsourcing Regional Outlook (USD Billion, 2019-2030)
-
North America
- US
- Canada
-
Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
-
Asia-Pacific
- China
- Japan
- India
- Australia
- South Korea
- Australia
- Rest of Asia-Pacific
-
Rest of the World
-
Middle East
-
Africa
-
Latin America
-
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.