Meningococcal Vaccines Market Overview:
Meningococcal Vaccines Market size is projected to be worth USD 6.80 billion by 2032, registering a CAGR of 7.3% during the forecast period (2023 - 2032), The market was valued USD 3.12 billion in 2021.
Meningococcal vaccinations are designed to protect against infection with Neisseria meningitidis. Vaccines targeting certain meningococcus strains, such as A, B, C, W-135, and Y, are available. In general, the vaccines produce immunity levels of 80 percent to 100 percent for two years, with some requiring booster doses as well. The vaccines might be monovalent, focused on a specific type of meningococcus, or multivalent in nature. The key factors that drive the global meningococcal vaccines market are the Increasing awareness and surge in travel vaccine, increase in R&D for vaccine technology. Increasing Inclusion in National Immunization Programs is expected to support the growth of the meningococcal vaccines market. However, the stringent regulatory guidelines and product recalls is anticipated to hamper the market growth.
COVID-19 Analysis
The COVID-19 pandemic has had a detrimental impact on the market for meningococcal vaccines. It greatly hampered meningococcal illness diagnosis, monitoring, and prevention. The pandemic's impact also exacerbated antibiotic resistance, which is the primary cause of the rise of antibiotic-resistant meningococcal strains. During the COVID-19 crisis, social distancing restrictions and steep cuts in consumers' discretionary budget reduced children meningococcal immunisation, perhaps leading to the resurgence of bacterial infections.
The lockdown and restrictions due to COVID-19 in several regions had resulted in a negative impact on the supply chain of the meningococcal vaccines market. As a result of the pandemic, several countries had implemented import and export restrictions, which adversely affected the supply chain of the meningococcal vaccine market.
Meningococcal Vaccines Market Dynamics
Market Drivers
- Increasing awareness and surge in travel vaccine
The increasing awareness about meningococcal vaccines and increasing antibiotics resistance of several Neisseria meningitidis clonal complexes is leading to a surge in immunization programs across the globe. For instance, several Neisseria meningitidis clonal complexes, particularly CC4821 and CC11, continue to exhibit resistance to antibiotics. In addition, increasing outbreaks of meningococcal diseases also drive the market. Moreover, an increasing number of travellers and the inclusion of the meningococcal vaccine as a mandatory requirement for a few countries are also driving the market.
- Increase in R&D for vaccine technology
Market Restraints
- Stringent regulatory guidelines and product recalls
Stringent regulatory guidelines on vaccine approval are among the major challenges in the market's growth. The US FDA and the EU European Medicines Agency (EMA) are the leading bodies involved in approving vaccines. All authorized vaccines are required to undergo a stringent regulatory approval process before they are marketed. These approvals ensure that vaccines meet safety, quality, and efficacy measures.
Market Opportunities
- Increasing Inclusion in National Immunization Programs
In several countries, meningococcal vaccines are included in routine immunization via national immunization programs. The Addition of meningococcal vaccines to national immunization programs with an aim to ensure vaccine supply sustainability and affordability in public and private sectors leads to significant opportunities for manufacturers. Thus, increasing the inclusion of meningococcal vaccines in national immunization programs is anticipated to provide significant growth opportunities for the meningococcal vaccine market.
Value Chain Analysis
The global meningococcal vaccine market is growing steadily and is expected to continue growing at the same rate in the near future. This is due to the growing contribution of existing players to make the product available to the public, and the rising prevalence of meningitis in children and increase in acceptance in several countries across the globe in their national immunization programs drive the market growth. The value chain analysis for the global meningococcal vaccine market comprises four major components: research and product development, manufacturing, distribution & sales, and post-sales monitoring.
Meningococcal Vaccines Market Segment Overview
Meningococcal Vaccines Vaccine Type Insights
A conjugate vaccine is a type of subunit vaccine which combines a weak antigen with a strong antigen as a carrier so that the immune system has a stronger response to the weak antigen. This segment's growth in the meningococcal vaccines market is because of its key advantages, such as long-lasting immunity and its ability to induce herd immunity.
The rise in prevalence of meningitis, increasing initiatives by government and regulatory authorities, and strong funding support from non-profit organizations are driving the growth of the meningococcal vaccines market in the subcapsular segment. In addition, increasing public health awareness initiatives to help prevent children, teens, and adolescents from getting infected by meningococcal disease point to an influx of opportunities for the market players. The increasing adoption of MenB vaccines is further expected to increase demand for this segment.
Polysaccharide vaccines can be multivalent or monovalent depending on the presence of capsular meningococcal polysaccharides in the vaccine. These vaccines stimulate a B-cell immune response resulting in a serotype-specific antibody production by the immune system, thus providing efficient immunity. The surge in immunization programs, increasing research and development for vaccine technology, and growing healthcare spending drive the growth of polysaccharide vaccines in the meningococcal vaccines market.
Meningococcal Vaccines Serotype Insights
The meningococcal conjugate vaccine (MenACWY) protects against four types of meningococcal bacteria (types A, C, W, and Y). For instance, in May 2018, “Defeating meningitis by 2030” was introduced at the World Health Assembly by Eastern Mediterranean and African regions to develop a strategic roadmap for meningitis prevention by prioritizing areas for research and enhanced control activities has been zooming in the focus on new formulations, such as a fully liquid presentation of tetravalent vaccine for MenACWY and Menveo.
The MenB vaccine is prepared from three major proteins found on the surface of most meningococcal bacteria, combined with the outer membrane of the 1 MenB strain. This vaccine protects against one of the most common types of meningococcal bacteria i.e., type B. CDC recommends routine MenB vaccination for people 10 years or older who are at high risk for meningococcal disease.
MenC vaccine gives protection against meningococcal disease (a major cause of meningitis) caused by group C Neisseria meningitidis bacteria. According to the National Meningitis Association (NMA), around 600–1,000 people in the U.S. get meningococcal disease each year, with 15% of those deaths due to a lack of awareness of the disease's symptoms. As a result, increased meningitis cases are expected to drive up demand for MenC vaccines.
Meningococcal outbreaks in several countries have led to an upsurge in demand for meningococcal vaccines. The government is taking initiatives to replace the current meningitis C vaccine with new combination vaccines such as MenAC to combat rising deaths due to meningitis. For instance, in May 2019, the Ministry of Health of Ireland approved the MenAC vaccine for thousands of children and adults in Ireland due to rising cases of meningitis. Such initiatives are anticipated to boost the revenue of new meningococcal vaccines, which drives the market growth.
MenA vaccine gives protection against meningococcal disease (a major cause of meningitis) caused by meningococcus group A only. Healthcare professional training, patient information, support, and demand are driven by a significant increase in routine immunization. Companies indulging in the meningococcal vaccines market by forming strategic alliances and dominating the market, such as Serum Institute, offer Meningococcal A Conjugate Vaccine (Freeze-Dried).
Meningococcal Vaccines End user Insights
The high growth of the hospitals and clinics segment is attributed to the rising demand for vaccines and the growing prevalence and incidence of the meningococcal disease. Growing government and private initiatives toward developing meningococcal vaccines and gauging the potential demand for these vaccines positively contribute to the segment growth.
- Research & Academic Institutes
Research & academic institutes is anticipated to record the highest CAGR from 2022 to 2030. This is due to the increasing prevalence of the meningococcal disease among young children, adolescents, and growing research & academic institutes across the globe. Additionally, R&D activities and improvements in the healthcare infrastructure and in the research & academic institutes contribute to market growth.
The other segment includes retail pharmacies and travel clinics. The role of pharmacists in immunization and vaccination varies across the world. Therefore, in some countries, pharmacists are primarily involved in ensuring the safe supply and dispensing of vaccines, as well as advocating for immunization, while in other countries, they are empowered to play a more active role, as they are legally authorized to administer vaccination, manage patient’s vaccinations schedules organize vaccinations activities and campaigns.
Meningococcal Vaccines Market Regional Analysis
Global: Meningococcal Vaccines MARKET SHARE (%), BY Region, 2021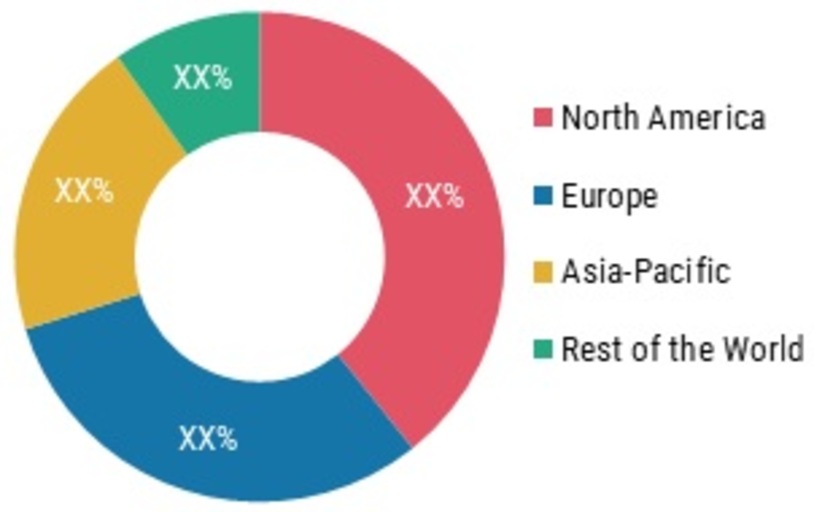 North America
North America
The North America dominated the global meningococcal vaccines market in 2021. This is due to the presence of key players, well-established healthcare infrastructure, favorable government initiatives for the vaccine development and distribution, and an increase in the number of R&D partnerships. Furthermore, preteens, teenagers, and young adults account for 21% of all meningococcal illness cases in the US are likely to positively impact the meningococcal vaccination market.
Europe
The meningococcal vaccine market in Europe is being driven by non-profit organizations that provide financing for research projects, healthcare professional training, patient information and support, and demand driven by a significant increase in routine immunization.
According to the European Centre for Disease Prevention and Control, invasive meningococcal disease (IMD) is a leading cause of meningitis and septicemia, with an estimated 200,000 cases occurring each year. The disease often progresses rapidly with an 8–15% case-fatality ratio. Certain European countries that are strategizing for a meningococcal vaccination program are boosting the meningococcal vaccine market.
Europe: Meningococcal Vaccines MARKET SHARE (%), BY country 2021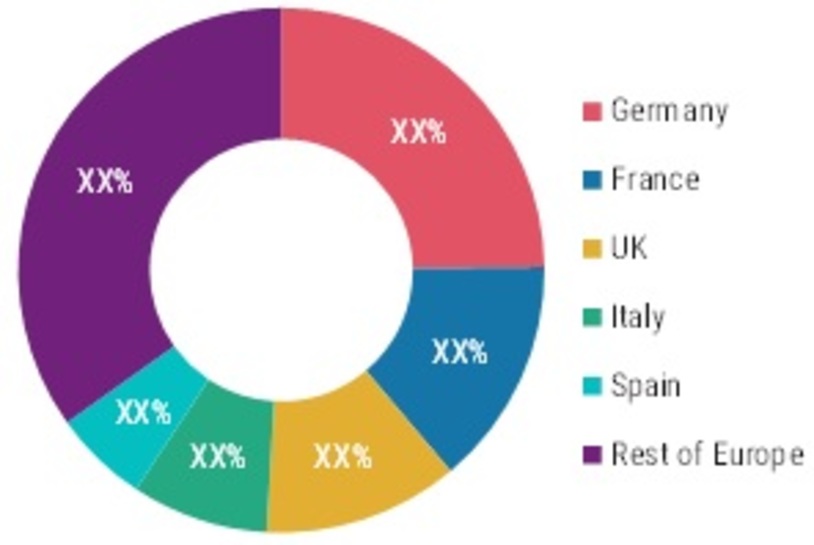 Asia-Pacific
Asia-Pacific
The Asia-Pacific region is expected to show the highest CAGR from 2021 to 2030. This is due to the increased transmission of meningococcal bacteria which has resulted in a significant increase in the number of clinical programs for the effective development of meningococcal vaccines due to unpredictable occurrences across different age groups and countries in the region. This has bolstered the Asia-Pacific meningococcal vaccines market.
Rest of the World
The Rest of the World comprises the Middle East, Africa, and Latin America. The market in rest of the world region is predicted to register a strong CAGR from 2022 to 2030. Meningococcal meningitis is found worldwide, but the disease has the highest prevalence in Africa. According to the African Centers for Disease Control and Prevention, Sub-Saharan Africa stretches from Senegal in the west to Ethiopia in the east. As a result, the high prevalence of meningococcal disease in the African region is on the rise. This led to the high demand for vaccines, further influencing the growth of the meningococcal vaccines market.
Meningococcal Vaccines Market Competitive Landscape
The global meningococcal vaccines market is extremely competitive, with players competing, partnering, and investing heavily in R&D to gain a significant market share. The market is moderately fragmented with rising competition, product launches, increasing collaborative partnerships, and other strategic decisions to achieve operational efficiency. The key players operating in the global meningococcal vaccines market include Pfizer Inc (US), Novartis AG (Switzerland), Sanofi S.A.(France), Serum Institute of India Pvt. Ltd (India), GlaxoSmithKline plc (UK), Merck & Co., Inc (US), Incepta Pharmaceuticals Ltd (Bangladesh), BIO-MED (US), Walvax Biotechnology Co., Ltd (China), Bio-Manguinhos (Brazil).
Pfizer, Inc. (Pfizer) is one of the leading research-based biopharmaceutical organizations. The company develops medicines and vaccines for a wide range of medical disciplines, including immunology, oncology, cardiology, diabetology/endocrinology, and neurology. Pfizer additionally teams up with healthcare providers, governments, and local communities to support and expand access to reliable and affordable healthcare worldwide. It operates in Germany, France, Greece, South Korea, Mexico, Russia, the Netherlands, India, Poland, Turkey, Israel, and China. The company has R&D platforms in biosimilars, gene therapy, precision medicines, and medicinal sciences. Some of its meningococcal vaccine products include Trumenba and NeisVac-C.
List of companies with HQ
- Pfizer Inc (US)
- Novartis AG (Switzerland)
- Sanofi S.A.(France)
- Serum Institute of India Pvt. Ltd (India)
- GlaxoSmithKline plc (UK)
- Merck & Co., Inc (US)
- Incepta Pharmaceuticals Ltd (Bangladesh)
- BIO-MED (US)
- Walvax Biotechnology Co., Ltd (China)
- Bio-Manguinhos (Brazil)
Recent Developments
- In May 2021, Bio-Manguinhos and Emergex (UK) have signed an agreement. This agreement establishes the framework for future clinical trials, production of meningococcal vaccine, COVID-19 vaccine, sales and marketing, and distribution within Brazil’s national health service.
- In November 2020, Sanofi S.A. received EU approval for MenQuadfi, a fully-liquid meningococcal vaccine. The vaccine is targeted towards individuals aged 12 and above.
- In April 2020, Sanofi S.A. received FDA approval for MenQuadfi, a meningococcal (MenACWY) vaccine in the US. This vaccination helps protect an enlarged age group and generates a robust immune response across various ages.
- In April 2018, Pfizer Inc announced that Trumenbas (Meningococcal Group B Vaccine) has achieved FDA breakthrough therapy designation for active immunization to prevent invasive illness caused by Neisseria meningitidis Group B (MenB) in children aged 1 to 9 years.
Report overview
The study covers the existing short-term and long-term market effects. It helps decision-makers to draught short-term and long-term business plans by region. The report covers major regions in North America, Europe, Asia Pacific, and Rest of the World. The report analyzes market drivers, restraints, opportunities, challenges, Porter's five forces, value chain, and impact of COVID-19 on the market.
Study Objectives
- To provide a comprehensive analysis of the global meningococcal vaccines market and its sub-segments, thereby providing a detailed structure of the industry
- To provide detailed insights into factors driving and restraining the growth of the global meningococcal vaccines market
- To estimate the market size of the global meningococcal vaccines market where 2018 to 2019 would be the historical period, 2021 shall be the base year, and 2022 to 2030 will be forecast period for the study
- To provide strategic profiling of key companies (manufacturers and distributors) present across the countries, and comprehensively analyze their competitiveness/competitive landscape in this market
- To provide a value chain analysis for the global meningococcal vaccines market.
Market Segmentation:
Global Meningococcal Vaccines Market, Vaccine Type Outlook
- Conjugate
- Subcapsular
- Polysaccharide
Global Meningococcal Vaccines Market, Serotype Outlook
- MenACWY
- MenB/BC
- MenC
- MenAC
- MenA
Global Meningococcal Vaccines Market, End user Outlook
- Hospitals
- Research & Academic Institutes
- Others
Global Meningococcal Vaccines Market, Region Outlook
- North America
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia-Pacific
- Rest of the World
- Middle East
- Africa
- Latin America
Intended audience:
- healthcare Institutions
- Product developers
- Manufacturers/Distributors
- Regulators and funding agencies
- Research institutes
| Report Attribute/Metric |
Details |
| Market Size |
USD 6.80 Billion |
| CAGR |
7.3% |
| Base Year |
2021 |
| Forecast Period |
2023-2032 |
| Historical Data |
2020 |
| Forecast Units |
Value (USD Billion) |
| Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered |
Vaccine, Serotype, End User and Region |
| Geographies Covered |
North America, Europe, Asia Pacific, and Rest of the World |
| Key Vendors |
Pfizer Inc (US), Novartis AG (Switzerland), Sanofi S.A.(France), Serum Institute of India Pvt. Ltd (India), GlaxoSmithKline plc (UK), Merck & Co., Inc (US), Incepta Pharmaceuticals Ltd (Bangladesh), BIO-MED (US), Walvax Biotechnology Co., Ltd (China), Bio-Manguinhos (Brazil) |
| Key Market Opportunities |
Increasing inclusion in national immunization programs |
| Key Market Drivers |
Increasing awareness and surge in travel vaccine Increase in R&D for vaccine technology |
Meningococcal Vaccines Market Highlights:
Frequently Asked Questions (FAQ) :
Meningococcal Vaccines Market size is expected to reach USD 6.80 billion by 2032
Pfizer Inc., Baxter International, Novartis Pharmaceuticals Canada Inc., JN-International Medical Corporation, Sanofi S.A., Biomed Pvt. Ltd., GlaxoSmithKline, and Serum Institute of India Ltd., are some of the major players operating in the Global Meningococcal Vaccines Market.
The market of Meningococcal Vaccines was valued USD 3,125.27 million in 2021
North America holds the largest share in the Global Meningococcal Vaccines Market.
Strategic initiatives such as mergers & acquisitions, collaborations, expansion, and technology/ product launch are some of the growth strategies that players operating in the global meningococcal vaccines market adopt to gain a larger competitive advantage.





 North America
North America Asia-Pacific
Asia-Pacific